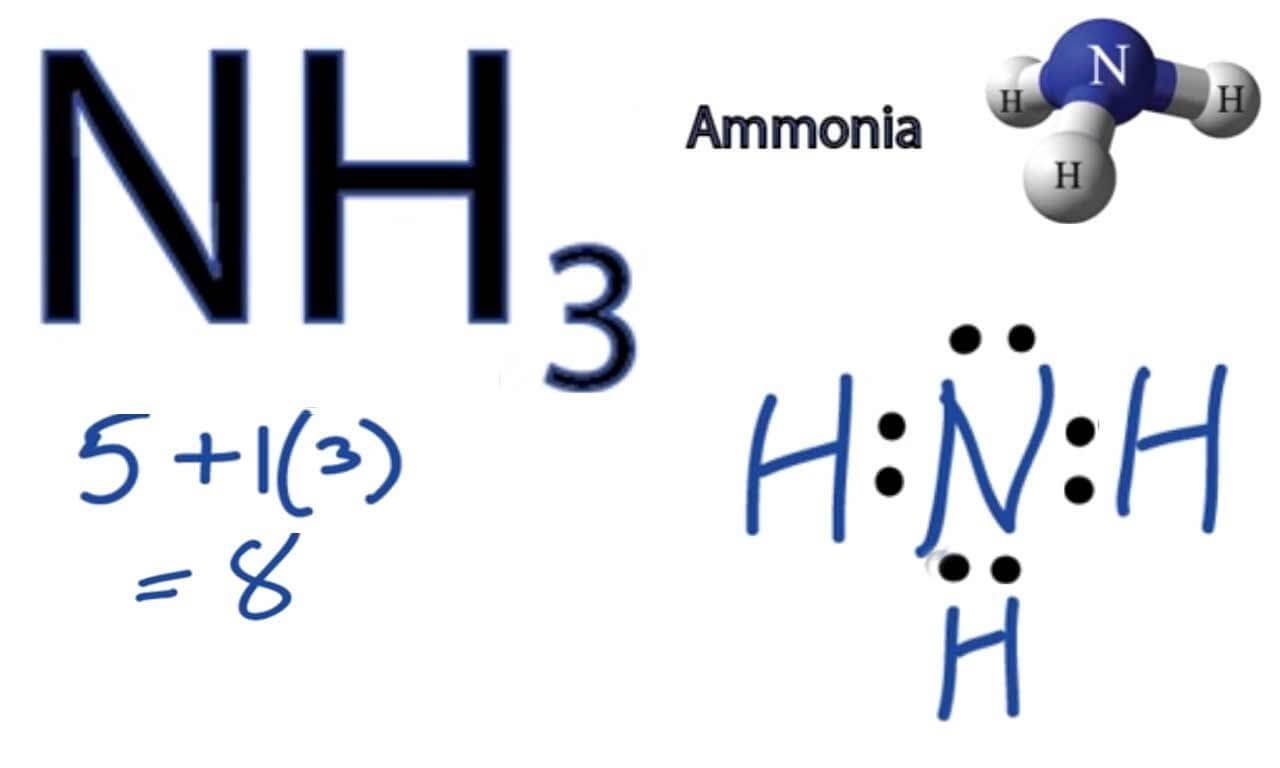What Is The Bond Angle Of Nh3
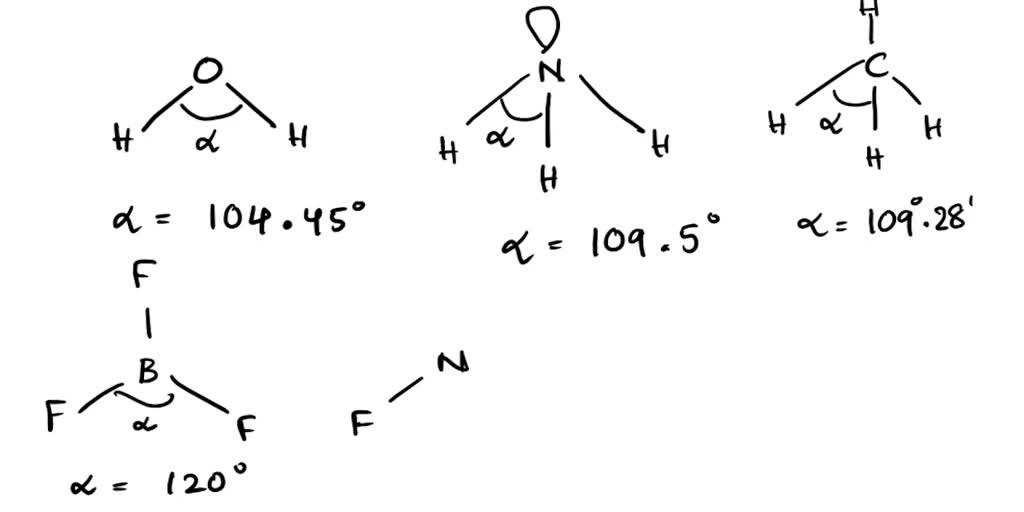
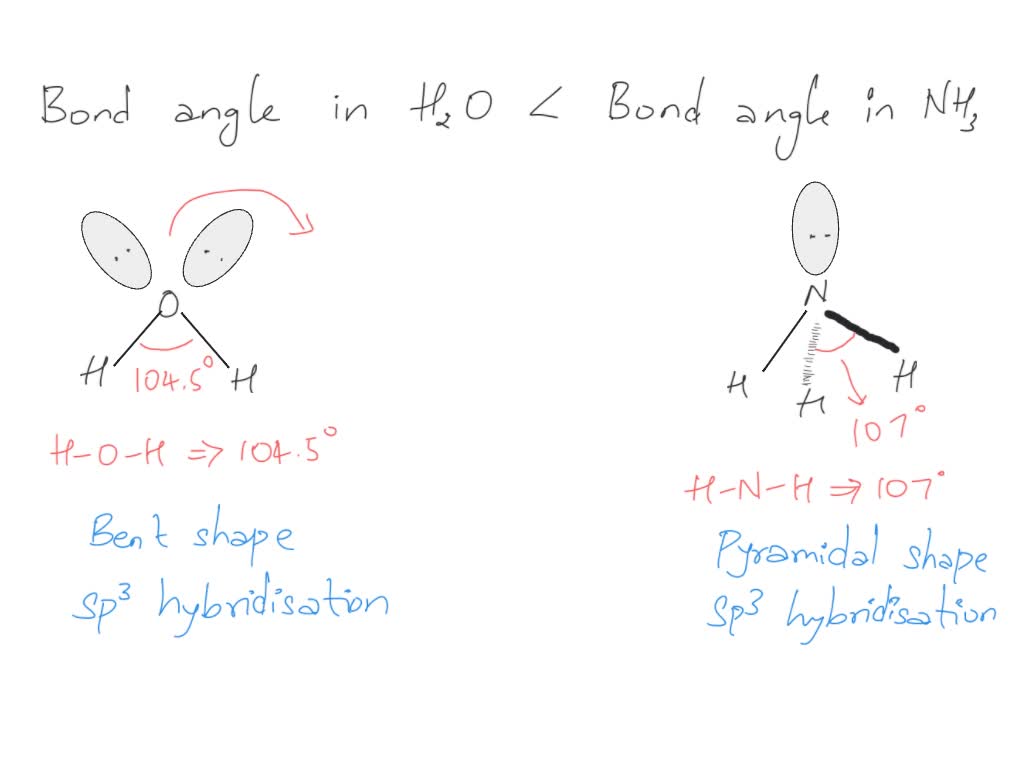
What Is The Bond Angle Of Nh3 - Both n h 3 and h 2 o are s p 3 hybridised, so the bond angle should be 109.5 ∘. The bond angle in a molecule of ammonia (nh3) is 107 degrees so why, when part of a transition metal complex is the bond angle 109.5 degrees. The ideal bond angle for. The bond angle of nh 3. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral. We know, the molecular geometry of nh 3 is a trigonal pyramid or distorted tetrahedral. In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. But n h 3 has one lone pair and water has two lones pairs. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb.
The ideal bond angle for. The bond angle in a molecule of ammonia (nh3) is 107 degrees so why, when part of a transition metal complex is the bond angle 109.5 degrees. The bond angle of nh 3. But n h 3 has one lone pair and water has two lones pairs. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. We know, the molecular geometry of nh 3 is a trigonal pyramid or distorted tetrahedral. Both n h 3 and h 2 o are s p 3 hybridised, so the bond angle should be 109.5 ∘.
The bond angle of nh 3. Both n h 3 and h 2 o are s p 3 hybridised, so the bond angle should be 109.5 ∘. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. The ideal bond angle for. We know, the molecular geometry of nh 3 is a trigonal pyramid or distorted tetrahedral. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral. The bond angle in a molecule of ammonia (nh3) is 107 degrees so why, when part of a transition metal complex is the bond angle 109.5 degrees. But n h 3 has one lone pair and water has two lones pairs. In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,.
Comparison of bond angle parameters Download Scientific Diagram
Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. We know, the molecular geometry of nh 3 is a trigonal pyramid or distorted tetrahedral. The ideal bond angle for. In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. The expected bond angle is 109.28 degrees, and the.
ii) Bond angle of NH3 is than H2O. Justify
Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. Both n h 3 and h 2 o are s p 3 hybridised, so the bond angle should be 109.5 ∘. We know, the molecular geometry of nh 3.
SOLVED Compare the bond angle predicted from VSEPR Theory and the bond
The ideal bond angle for. But n h 3 has one lone pair and water has two lones pairs. Both n h 3 and h 2 o are s p 3 hybridised, so the bond angle should be 109.5 ∘. We know, the molecular geometry of nh 3 is a trigonal pyramid or distorted tetrahedral. Nh_3 has a bond angle.
Nh3 Lewis Structure Bond Angle Drawing Easy
Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. Both n h 3 and h 2 o are s p 3 hybridised, so the bond angle should be 109.5 ∘. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral. The bond angle in a molecule of.
NH3 Molecular Geometry, Hybridization, Bond Angle and Molecular Shape
Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. Both n h 3 and h 2 o are s p 3 hybridised, so the bond angle should be 109.5 ∘. We know, the molecular geometry of nh 3 is a trigonal pyramid or distorted tetrahedral. The bond angle in.
SOLVED Bond angle in nh3 is greater than bond angle in a s h 3
But n h 3 has one lone pair and water has two lones pairs. Both n h 3 and h 2 o are s p 3 hybridised, so the bond angle should be 109.5 ∘. We know, the molecular geometry of nh 3 is a trigonal pyramid or distorted tetrahedral. The expected bond angle is 109.28 degrees, and the geometry.
Trigonal Pyramidal Bond Angle
In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. We know, the molecular geometry of nh 3 is a trigonal pyramid or distorted tetrahedral. The ideal bond angle for. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral. But n h 3 has one lone pair and water has two lones pairs.
Decreasing order of bond angle of (NH3 ,PH3 ,AsH3 ) Filo
Both n h 3 and h 2 o are s p 3 hybridised, so the bond angle should be 109.5 ∘. The bond angle of nh 3. But n h 3 has one lone pair and water has two lones pairs. The bond angle in a molecule of ammonia (nh3) is 107 degrees so why, when part of a transition.
Which has highest bond angle
The expected bond angle is 109.28 degrees, and the geometry is tetrahedral. In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. The bond angle of nh 3. We know, the molecular geometry of nh 3 is a trigonal.
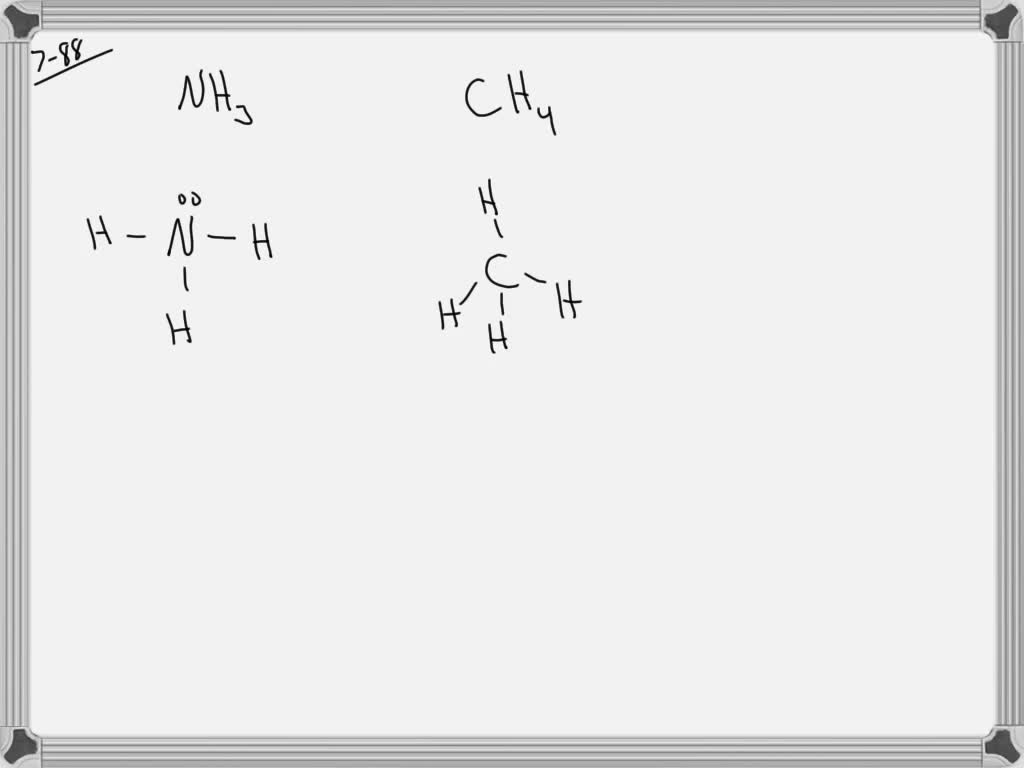
SOLVEDWhy is the HNH angle in NH3 smaller than the HCH bond angle
The expected bond angle is 109.28 degrees, and the geometry is tetrahedral. We know, the molecular geometry of nh 3 is a trigonal pyramid or distorted tetrahedral. The bond angle of nh 3. In contrast, ammonia shows trigonal pyramidal geometry and <109 bond angle,. Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about.
In Contrast, Ammonia Shows Trigonal Pyramidal Geometry And <109 Bond Angle,.
Nh_3 has a bond angle of about 106.67^@, while ph_3 has a bond angle of about 93.3^@, according to cccbdb. The bond angle of nh 3. The expected bond angle is 109.28 degrees, and the geometry is tetrahedral. Both n h 3 and h 2 o are s p 3 hybridised, so the bond angle should be 109.5 ∘.
The Ideal Bond Angle For.
We know, the molecular geometry of nh 3 is a trigonal pyramid or distorted tetrahedral. The bond angle in a molecule of ammonia (nh3) is 107 degrees so why, when part of a transition metal complex is the bond angle 109.5 degrees. But n h 3 has one lone pair and water has two lones pairs.