What Is The Bond Order Of Be2
What Is The Bond Order Of Be2 - Overall, the molecular orbital diagram for be2 shows a stable bonding. Bond order is equal to half of the difference between the number of electrons in bonding (\[{{n}_{b}}\]) and antibonding molecular orbitals (\[{{n}_{a}}\]). Hence, the bond order for b e 2 is 1 2 (n b − n a) where, n b =. The molecular orbital electronic configuration for be2 molecule can be written as:
Bond order is equal to half of the difference between the number of electrons in bonding (\[{{n}_{b}}\]) and antibonding molecular orbitals (\[{{n}_{a}}\]). Hence, the bond order for b e 2 is 1 2 (n b − n a) where, n b =. The molecular orbital electronic configuration for be2 molecule can be written as: Overall, the molecular orbital diagram for be2 shows a stable bonding.
Hence, the bond order for b e 2 is 1 2 (n b − n a) where, n b =. Overall, the molecular orbital diagram for be2 shows a stable bonding. The molecular orbital electronic configuration for be2 molecule can be written as: Bond order is equal to half of the difference between the number of electrons in bonding (\[{{n}_{b}}\]) and antibonding molecular orbitals (\[{{n}_{a}}\]).
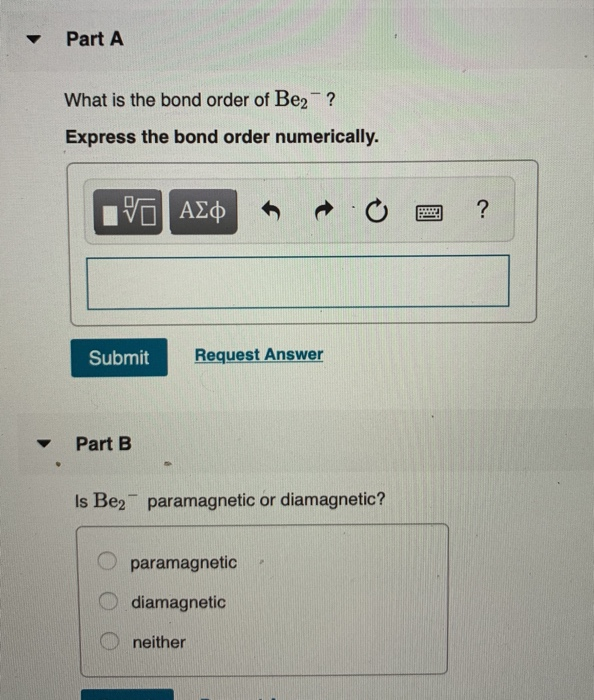
Solved Part A What is the bond order of Be2? Express the
Hence, the bond order for b e 2 is 1 2 (n b − n a) where, n b =. The molecular orbital electronic configuration for be2 molecule can be written as: Overall, the molecular orbital diagram for be2 shows a stable bonding. Bond order is equal to half of the difference between the number of electrons in bonding (\[{{n}_{b}}\]).
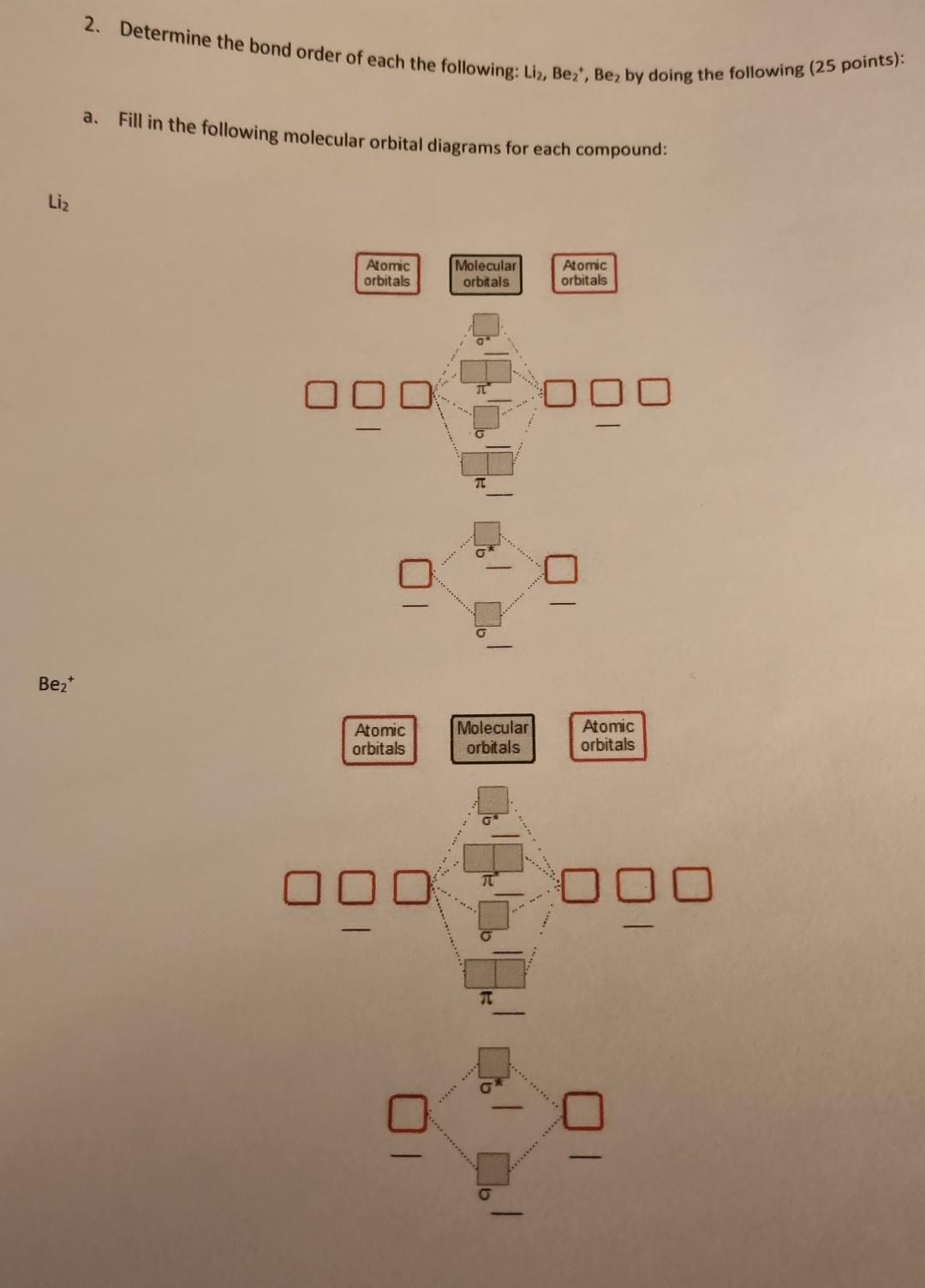
Solved 2. Determine the bond order nf an.L.. pints)b.
The molecular orbital electronic configuration for be2 molecule can be written as: Overall, the molecular orbital diagram for be2 shows a stable bonding. Bond order is equal to half of the difference between the number of electrons in bonding (\[{{n}_{b}}\]) and antibonding molecular orbitals (\[{{n}_{a}}\]). Hence, the bond order for b e 2 is 1 2 (n b − n.
Find out the bond order ofBe2
Hence, the bond order for b e 2 is 1 2 (n b − n a) where, n b =. The molecular orbital electronic configuration for be2 molecule can be written as: Bond order is equal to half of the difference between the number of electrons in bonding (\[{{n}_{b}}\]) and antibonding molecular orbitals (\[{{n}_{a}}\]). Overall, the molecular orbital diagram for.
resonance Determining the bond order Chemistry Stack Exchange
Hence, the bond order for b e 2 is 1 2 (n b − n a) where, n b =. The molecular orbital electronic configuration for be2 molecule can be written as: Overall, the molecular orbital diagram for be2 shows a stable bonding. Bond order is equal to half of the difference between the number of electrons in bonding (\[{{n}_{b}}\]).
(a) What are the relationships among bond order, bond length, and bond
The molecular orbital electronic configuration for be2 molecule can be written as: Bond order is equal to half of the difference between the number of electrons in bonding (\[{{n}_{b}}\]) and antibonding molecular orbitals (\[{{n}_{a}}\]). Hence, the bond order for b e 2 is 1 2 (n b − n a) where, n b =. Overall, the molecular orbital diagram for.
Solved Part A What is the bond order of Be2? Express the
Bond order is equal to half of the difference between the number of electrons in bonding (\[{{n}_{b}}\]) and antibonding molecular orbitals (\[{{n}_{a}}\]). The molecular orbital electronic configuration for be2 molecule can be written as: Hence, the bond order for b e 2 is 1 2 (n b − n a) where, n b =. Overall, the molecular orbital diagram for.
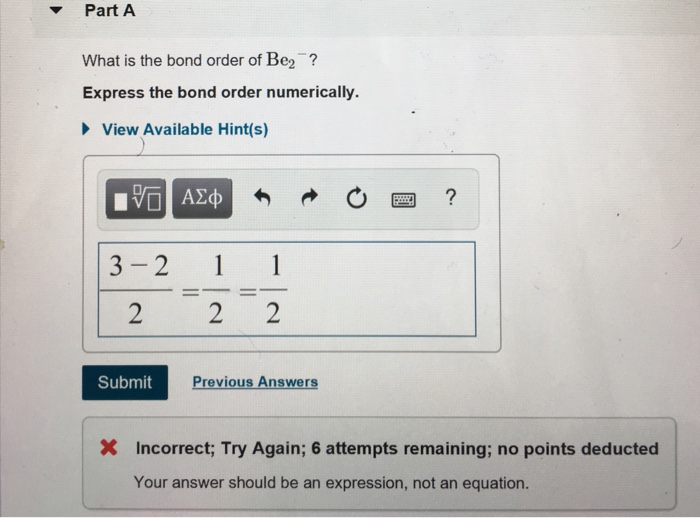
SOLVED What is the bond order of Bez Express the bond order
Hence, the bond order for b e 2 is 1 2 (n b − n a) where, n b =. Bond order is equal to half of the difference between the number of electrons in bonding (\[{{n}_{b}}\]) and antibonding molecular orbitals (\[{{n}_{a}}\]). The molecular orbital electronic configuration for be2 molecule can be written as: Overall, the molecular orbital diagram for.
Solved 2. Determine the bond order nf an.L.. pints)b.
Hence, the bond order for b e 2 is 1 2 (n b − n a) where, n b =. The molecular orbital electronic configuration for be2 molecule can be written as: Bond order is equal to half of the difference between the number of electrons in bonding (\[{{n}_{b}}\]) and antibonding molecular orbitals (\[{{n}_{a}}\]). Overall, the molecular orbital diagram for.
Solved 2. Determine the bond order nf an.L.. pints)b.
Overall, the molecular orbital diagram for be2 shows a stable bonding. The molecular orbital electronic configuration for be2 molecule can be written as: Bond order is equal to half of the difference between the number of electrons in bonding (\[{{n}_{b}}\]) and antibonding molecular orbitals (\[{{n}_{a}}\]). Hence, the bond order for b e 2 is 1 2 (n b − n.
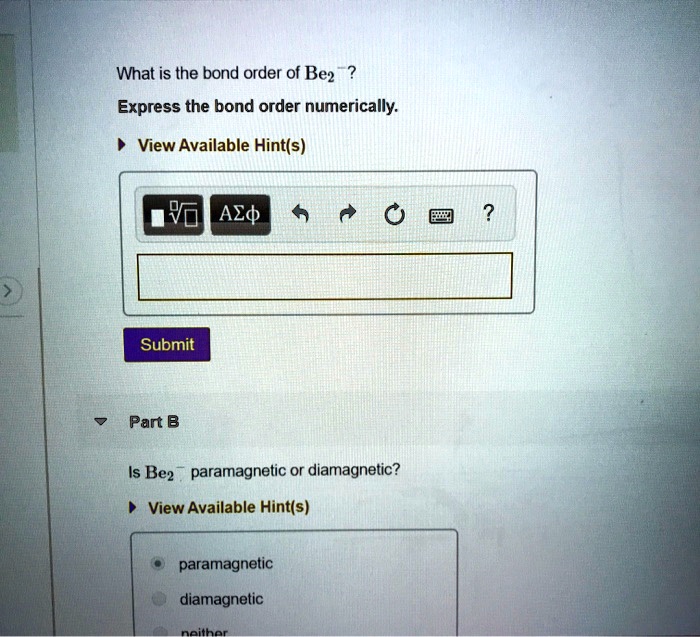
SOLVED What is the bond order?
Bond order is equal to half of the difference between the number of electrons in bonding (\[{{n}_{b}}\]) and antibonding molecular orbitals (\[{{n}_{a}}\]). The molecular orbital electronic configuration for be2 molecule can be written as: Hence, the bond order for b e 2 is 1 2 (n b − n a) where, n b =. Overall, the molecular orbital diagram for.
The Molecular Orbital Electronic Configuration For Be2 Molecule Can Be Written As:
Hence, the bond order for b e 2 is 1 2 (n b − n a) where, n b =. Overall, the molecular orbital diagram for be2 shows a stable bonding. Bond order is equal to half of the difference between the number of electrons in bonding (\[{{n}_{b}}\]) and antibonding molecular orbitals (\[{{n}_{a}}\]).