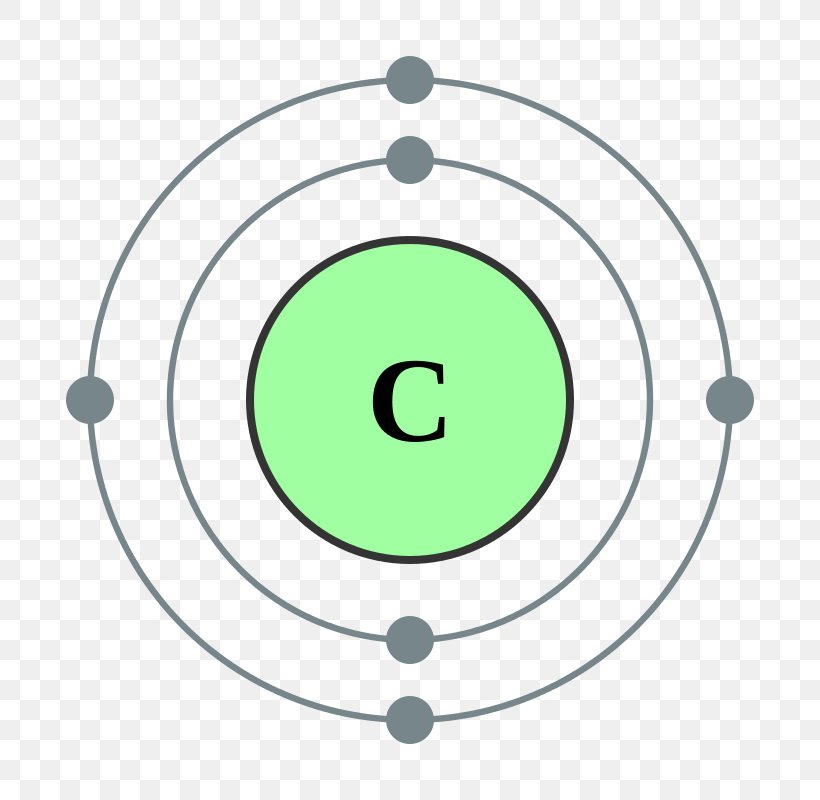
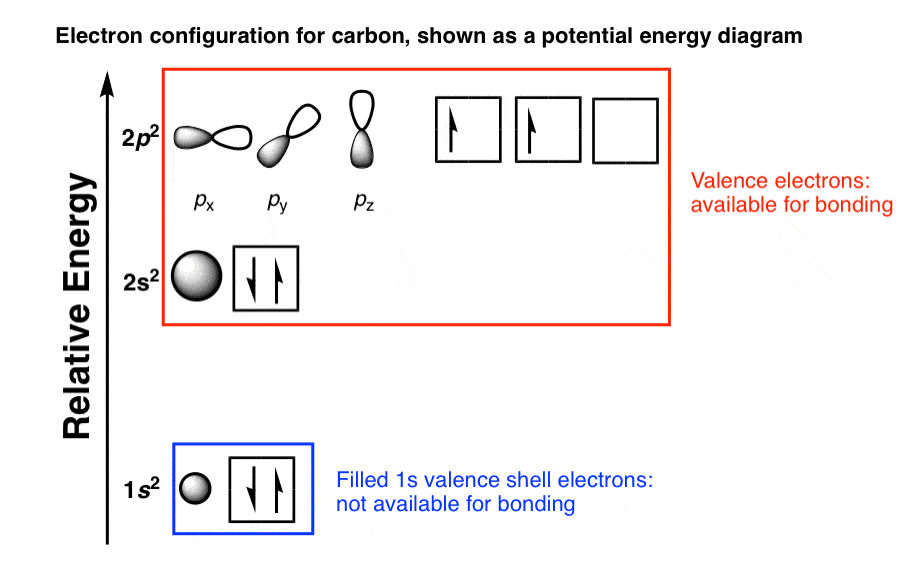
What Is The Electron Configuration Of Co2
What Is The Electron Configuration Of Co2 - To turn co into co2+ ion, we need to remove 2 electrons. The electron configuration of the co2 ion can be determined by. Its electron configuration is [\(\text{ar}\)] 3d^7 4s^2. To write the configuration for the cobalt ions, first we need to write the electron configuration for just cobalt (co). What is the electronic configuration of co atom and a co2+ ion ?? We will remove the 2 electrons from. We first need to find the. This ion has 18 electrons, two fewer than the neutral co molecule. The electron configuration of the co2 ion is 1s² 2s². The electron configuration of the co2+ ion is 1s2 2s2 2p6.
The electron configuration of the co2 ion can be determined by. The electron configuration of the co2 ion is 1s² 2s². We first need to find the. This ion has 18 electrons, two fewer than the neutral co molecule. Its electron configuration is [\(\text{ar}\)] 3d^7 4s^2. To turn co into co2+ ion, we need to remove 2 electrons. The electron configuration of the co2+ ion is 1s2 2s2 2p6. Co2+ would most likely be 1s22s22p63s23p64s03d7, and co would have. What is the electronic configuration of co atom and a co2+ ion ?? We will remove the 2 electrons from.
The electron configuration of the co2+ ion is 1s2 2s2 2p6. Its electron configuration is [\(\text{ar}\)] 3d^7 4s^2. We first need to find the. To turn co into co2+ ion, we need to remove 2 electrons. We will remove the 2 electrons from. The electron configuration of the co2 ion can be determined by. What is the electronic configuration of co atom and a co2+ ion ?? This ion has 18 electrons, two fewer than the neutral co molecule. The electron configuration of the co2 ion is 1s² 2s². To write the configuration for the cobalt ions, first we need to write the electron configuration for just cobalt (co).
SOLUTION Co2 part 3 electron configuration Studypool
The electron configuration of the co2 ion is 1s² 2s². What is the electronic configuration of co atom and a co2+ ion ?? The electron configuration of the co2+ ion is 1s2 2s2 2p6. Its electron configuration is [\(\text{ar}\)] 3d^7 4s^2. To turn co into co2+ ion, we need to remove 2 electrons.
Carbon electron configuration Stock Image C029/5022 Science Photo
What is the electronic configuration of co atom and a co2+ ion ?? To turn co into co2+ ion, we need to remove 2 electrons. The electron configuration of the co2+ ion is 1s2 2s2 2p6. Co2+ would most likely be 1s22s22p63s23p64s03d7, and co would have. We first need to find the.
Carbon Electron Configuration Diagram
To write the configuration for the cobalt ions, first we need to write the electron configuration for just cobalt (co). We will remove the 2 electrons from. What is the electronic configuration of co atom and a co2+ ion ?? To turn co into co2+ ion, we need to remove 2 electrons. The electron configuration of the co2 ion is.
SOLUTION Co2 part 3 electron configuration Studypool
The electron configuration of the co2 ion can be determined by. To turn co into co2+ ion, we need to remove 2 electrons. We will remove the 2 electrons from. We first need to find the. The electron configuration of the co2 ion is 1s² 2s².
Electron Configuration for Cobalt (Co and Co2+, Co3+)
The electron configuration of the co2+ ion is 1s2 2s2 2p6. This ion has 18 electrons, two fewer than the neutral co molecule. The electron configuration of the co2 ion can be determined by. We first need to find the. Co2+ would most likely be 1s22s22p63s23p64s03d7, and co would have.
Carbon Electron Configuration Diagram
We first need to find the. This ion has 18 electrons, two fewer than the neutral co molecule. The electron configuration of the co2+ ion is 1s2 2s2 2p6. What is the electronic configuration of co atom and a co2+ ion ?? Its electron configuration is [\(\text{ar}\)] 3d^7 4s^2.
Electron Configuration Electron Shell Valence Electron Carbon, PNG
The electron configuration of the co2 ion can be determined by. What is the electronic configuration of co atom and a co2+ ion ?? We will remove the 2 electrons from. Its electron configuration is [\(\text{ar}\)] 3d^7 4s^2. Co2+ would most likely be 1s22s22p63s23p64s03d7, and co would have.
Carbon electron configuration aufbau principle tatacycle
What is the electronic configuration of co atom and a co2+ ion ?? Its electron configuration is [\(\text{ar}\)] 3d^7 4s^2. To write the configuration for the cobalt ions, first we need to write the electron configuration for just cobalt (co). We first need to find the. The electron configuration of the co2+ ion is 1s2 2s2 2p6.
SOLUTION Co2 part 3 electron configuration Studypool
To write the configuration for the cobalt ions, first we need to write the electron configuration for just cobalt (co). This ion has 18 electrons, two fewer than the neutral co molecule. We will remove the 2 electrons from. The electron configuration of the co2+ ion is 1s2 2s2 2p6. To turn co into co2+ ion, we need to remove.
SOLUTION Co2 part 3 electron configuration Studypool
We first need to find the. We will remove the 2 electrons from. Co2+ would most likely be 1s22s22p63s23p64s03d7, and co would have. To write the configuration for the cobalt ions, first we need to write the electron configuration for just cobalt (co). To turn co into co2+ ion, we need to remove 2 electrons.
To Write The Configuration For The Cobalt Ions, First We Need To Write The Electron Configuration For Just Cobalt (Co).
To turn co into co2+ ion, we need to remove 2 electrons. We first need to find the. What is the electronic configuration of co atom and a co2+ ion ?? This ion has 18 electrons, two fewer than the neutral co molecule.
The Electron Configuration Of The Co2 Ion Is 1S² 2S².
Co2+ would most likely be 1s22s22p63s23p64s03d7, and co would have. The electron configuration of the co2+ ion is 1s2 2s2 2p6. We will remove the 2 electrons from. The electron configuration of the co2 ion can be determined by.