What Is The Electron Geometry Of H2S
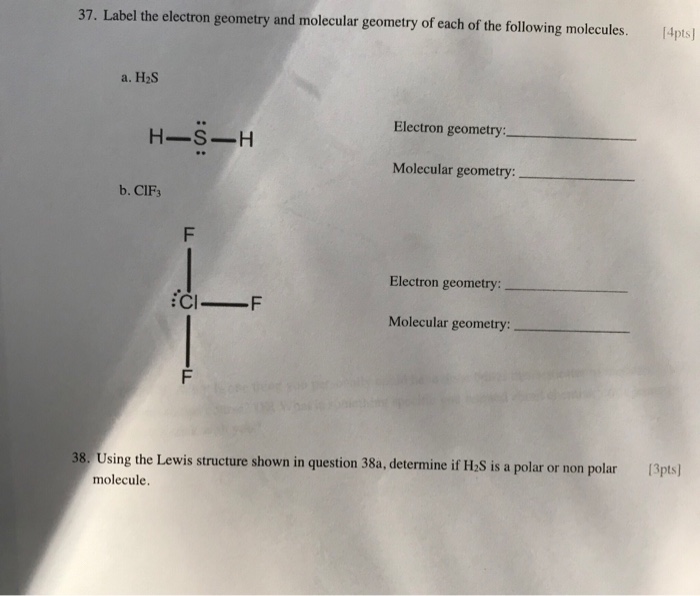
What Is The Electron Geometry Of H2S - The lewis structure of h2s indicates that it adopts a bent geometry, with a bond angle of approximately 104.5 degrees.
The lewis structure of h2s indicates that it adopts a bent geometry, with a bond angle of approximately 104.5 degrees.
The lewis structure of h2s indicates that it adopts a bent geometry, with a bond angle of approximately 104.5 degrees.
What is the electron geometry of \ce{CH3NH2}? Quizlet
The lewis structure of h2s indicates that it adopts a bent geometry, with a bond angle of approximately 104.5 degrees.
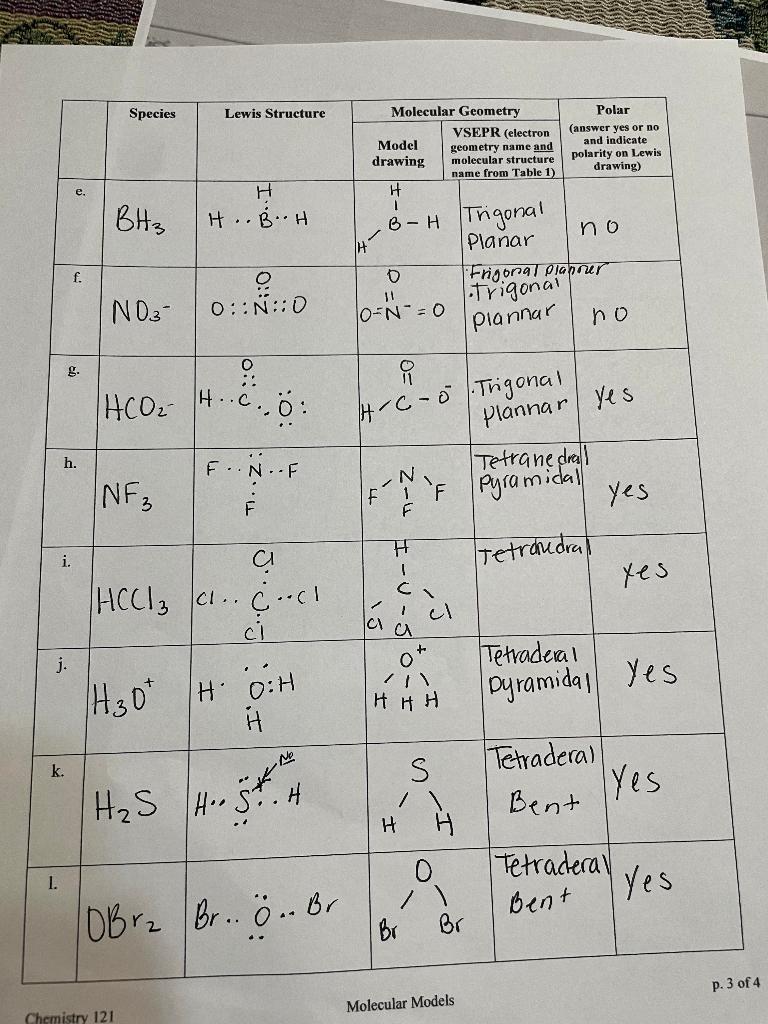
Solved Species Lewis Structure Molecular Geometry VSEPR
The lewis structure of h2s indicates that it adopts a bent geometry, with a bond angle of approximately 104.5 degrees.
Top Molecular Vs Electron Geometry Chart Background GM
The lewis structure of h2s indicates that it adopts a bent geometry, with a bond angle of approximately 104.5 degrees.
Determine the electron geometry (eg) and molecular geometry Quizlet
The lewis structure of h2s indicates that it adopts a bent geometry, with a bond angle of approximately 104.5 degrees.
Determine the electron geometry, molecular geometry and pola Quizlet
The lewis structure of h2s indicates that it adopts a bent geometry, with a bond angle of approximately 104.5 degrees.
Determine the electron geometry, molecular geometry and pola Quizlet
The lewis structure of h2s indicates that it adopts a bent geometry, with a bond angle of approximately 104.5 degrees.
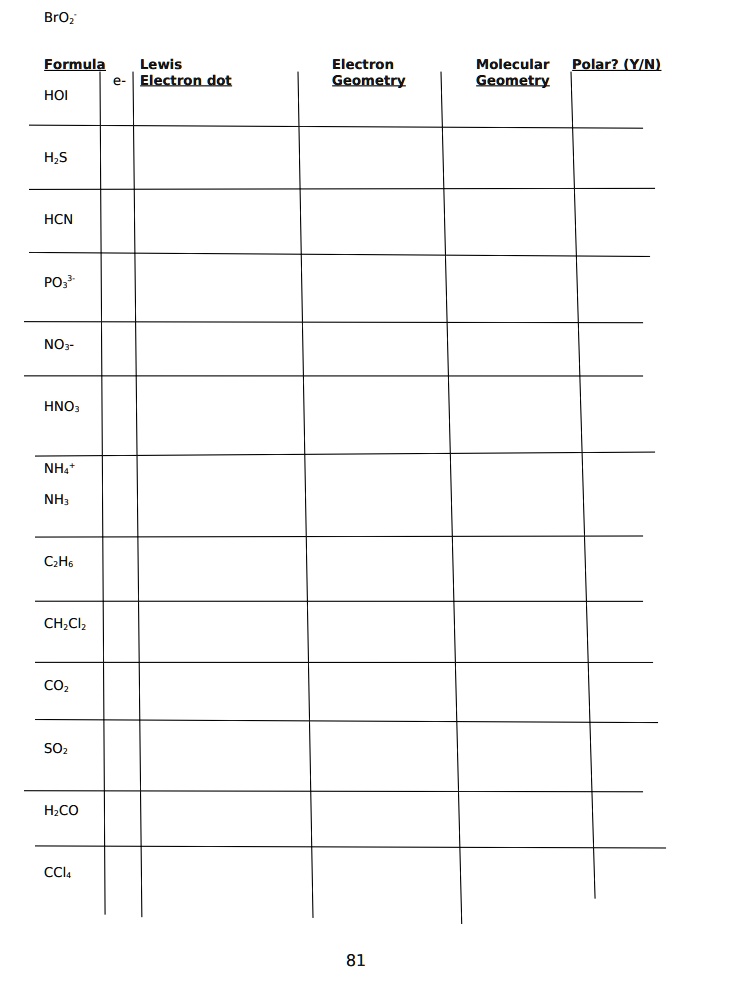
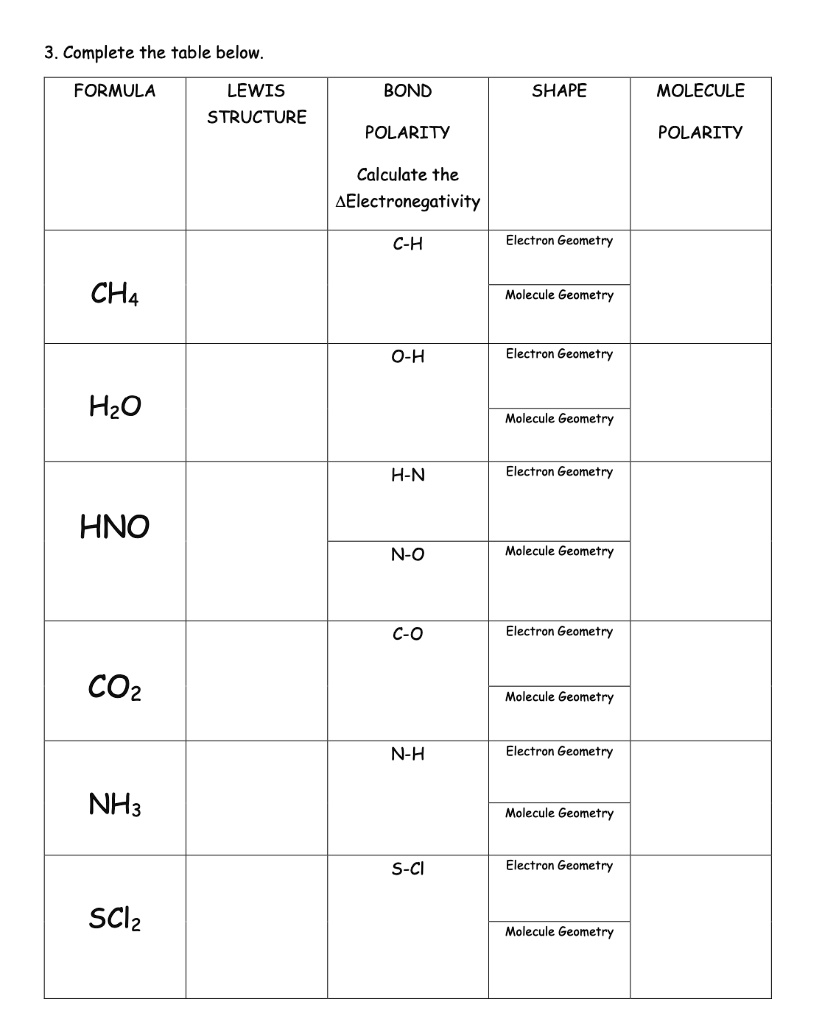
SOLVED the table below FORMULA LEWIS BOND SHAPE MOLECULE
The lewis structure of h2s indicates that it adopts a bent geometry, with a bond angle of approximately 104.5 degrees.
[Solved] Determine the electron geometry for each molecule
The lewis structure of h2s indicates that it adopts a bent geometry, with a bond angle of approximately 104.5 degrees.
Solved 37. Label the electron geometry and molecular
The lewis structure of h2s indicates that it adopts a bent geometry, with a bond angle of approximately 104.5 degrees.





![[Solved] Determine the electron geometry for each molecule](https://media.cheggcdn.com/media/c5c/c5cdeca8-cbd3-4610-812b-17f7e11d605b/phpkeMGn5)