What Is The Empirical Formula For Propene C3H6
What Is The Empirical Formula For Propene C3H6 - What is the empirical formula for propene, c3h6? This is found by dividing the subscripts of each element by their greatest common factor, which in this case is 3. The chemical formula of propene is c3h6. C3h6 is an empirical formula for propene. To find the percentage composition, calculate the molar mass of each element and then divide by the molar mass of propene. The empirical formula for propene (c3h6) is ch2. What is the properly written empirical formula of a compound of molecular formula c6h12 with a molar mass equal to 84.16 gram. The molecular formula is the true representation of the composition of a molecule showing the number. C3h6 is an empirical formula for propene.
The chemical formula of propene is c3h6. What is the empirical formula for propene, c3h6? The molecular formula is the true representation of the composition of a molecule showing the number. C3h6 is an empirical formula for propene. To find the percentage composition, calculate the molar mass of each element and then divide by the molar mass of propene. This is found by dividing the subscripts of each element by their greatest common factor, which in this case is 3. What is the properly written empirical formula of a compound of molecular formula c6h12 with a molar mass equal to 84.16 gram. C3h6 is an empirical formula for propene. The empirical formula for propene (c3h6) is ch2.
To find the percentage composition, calculate the molar mass of each element and then divide by the molar mass of propene. C3h6 is an empirical formula for propene. What is the properly written empirical formula of a compound of molecular formula c6h12 with a molar mass equal to 84.16 gram. The empirical formula for propene (c3h6) is ch2. The chemical formula of propene is c3h6. The molecular formula is the true representation of the composition of a molecule showing the number. What is the empirical formula for propene, c3h6? This is found by dividing the subscripts of each element by their greatest common factor, which in this case is 3. C3h6 is an empirical formula for propene.
Molecular Formula And Empirical Formula Worksheet Printable Calendars
C3h6 is an empirical formula for propene. The molecular formula is the true representation of the composition of a molecule showing the number. What is the properly written empirical formula of a compound of molecular formula c6h12 with a molar mass equal to 84.16 gram. This is found by dividing the subscripts of each element by their greatest common factor,.
Propene (propylene, methylethylene) is an unsaturated organic compound
This is found by dividing the subscripts of each element by their greatest common factor, which in this case is 3. To find the percentage composition, calculate the molar mass of each element and then divide by the molar mass of propene. C3h6 is an empirical formula for propene. What is the properly written empirical formula of a compound of.
What is the structural formula for propene?
The molecular formula is the true representation of the composition of a molecule showing the number. The empirical formula for propene (c3h6) is ch2. The chemical formula of propene is c3h6. What is the properly written empirical formula of a compound of molecular formula c6h12 with a molar mass equal to 84.16 gram. This is found by dividing the subscripts.
Propene Formula
What is the properly written empirical formula of a compound of molecular formula c6h12 with a molar mass equal to 84.16 gram. This is found by dividing the subscripts of each element by their greatest common factor, which in this case is 3. To find the percentage composition, calculate the molar mass of each element and then divide by the.
Propene Alchetron, The Free Social Encyclopedia
The chemical formula of propene is c3h6. The empirical formula for propene (c3h6) is ch2. What is the properly written empirical formula of a compound of molecular formula c6h12 with a molar mass equal to 84.16 gram. C3h6 is an empirical formula for propene. To find the percentage composition, calculate the molar mass of each element and then divide by.
Solved (c) Propene and but 2 ene are alkenes. (i) Draw the displayed
What is the properly written empirical formula of a compound of molecular formula c6h12 with a molar mass equal to 84.16 gram. C3h6 is an empirical formula for propene. The chemical formula of propene is c3h6. What is the empirical formula for propene, c3h6? This is found by dividing the subscripts of each element by their greatest common factor, which.
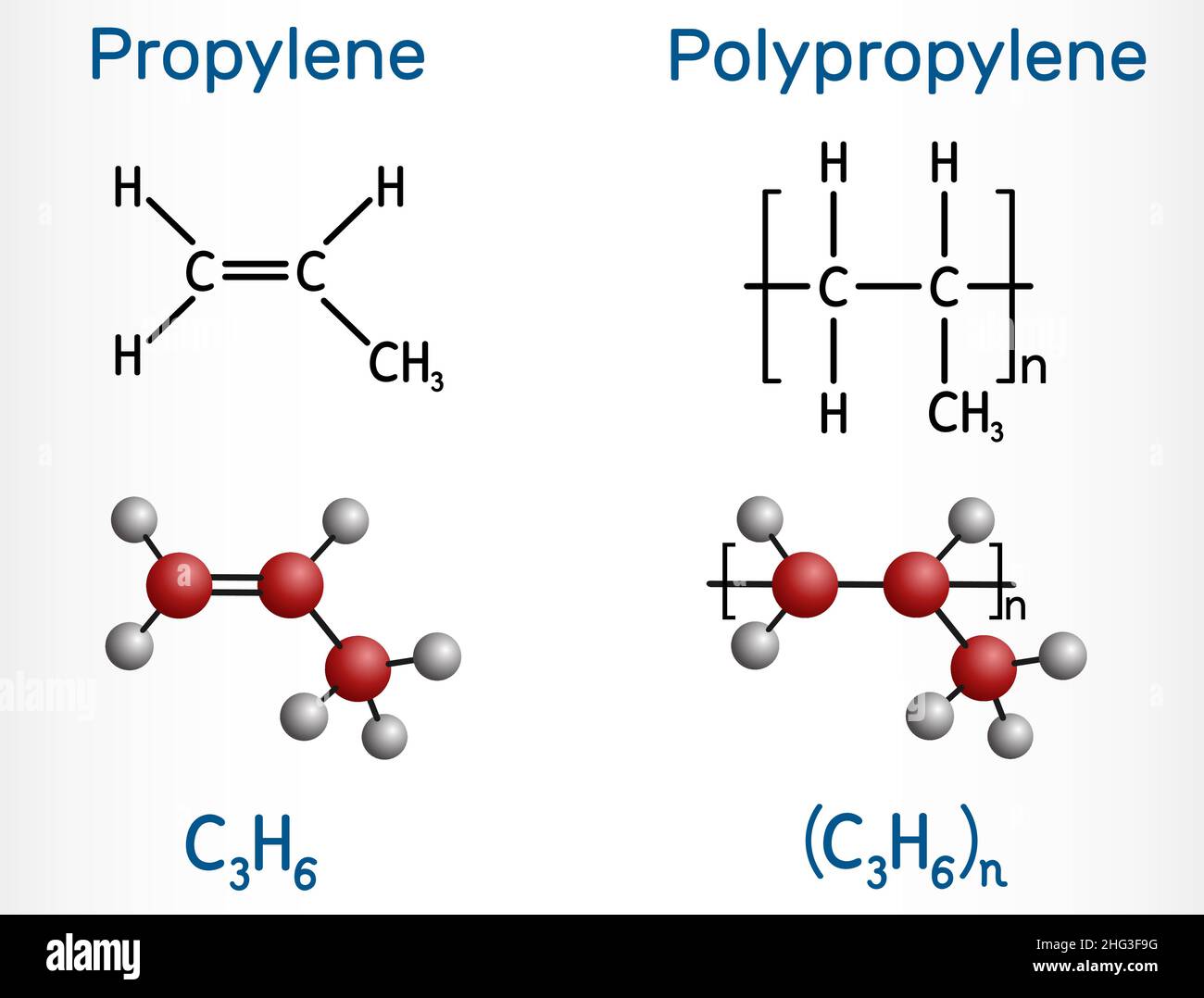
Propene C3H6 Molecular Geometry Hybridization Molecular Weight
The chemical formula of propene is c3h6. What is the properly written empirical formula of a compound of molecular formula c6h12 with a molar mass equal to 84.16 gram. What is the empirical formula for propene, c3h6? The empirical formula for propene (c3h6) is ch2. This is found by dividing the subscripts of each element by their greatest common factor,.
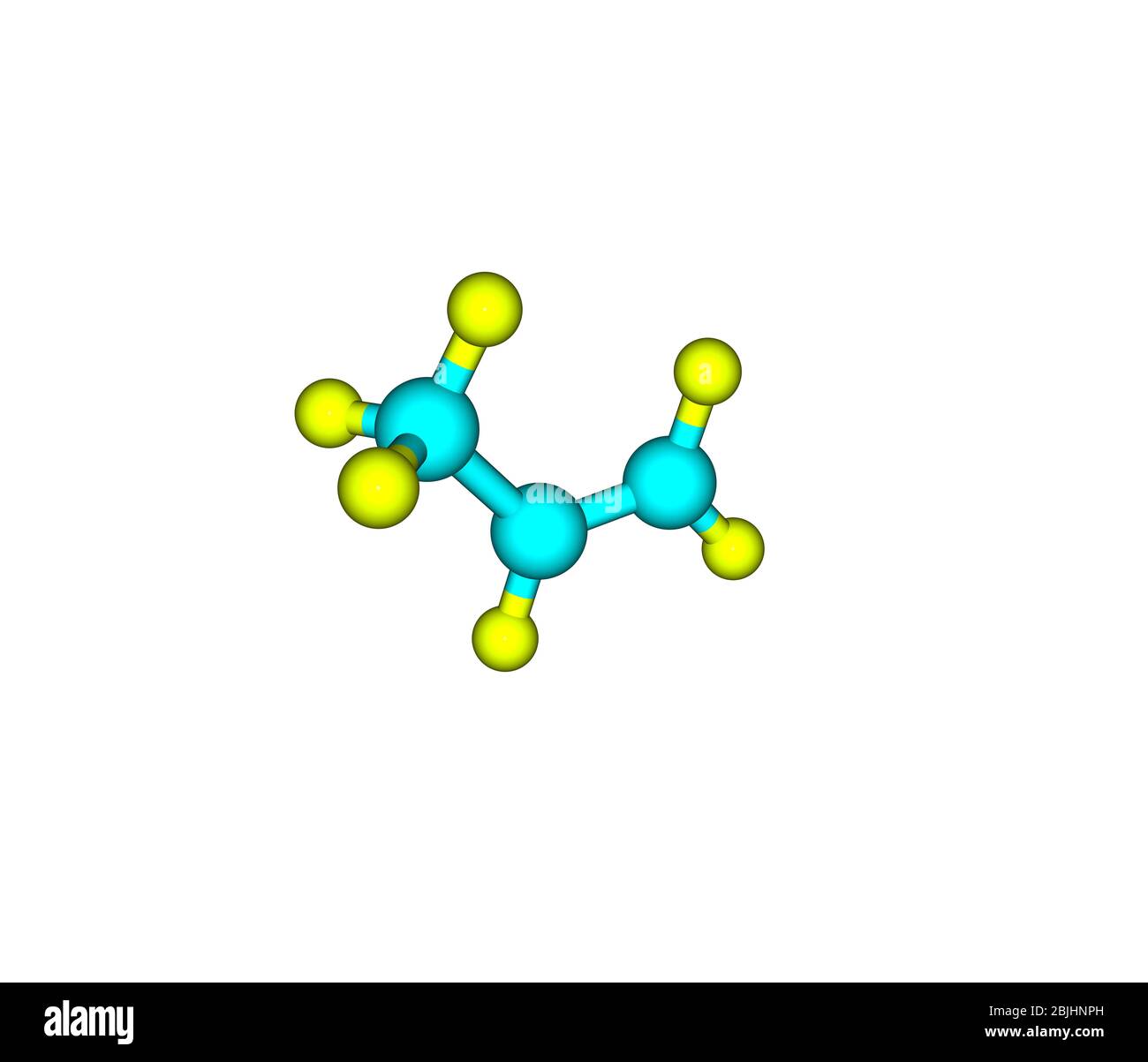
Propene molecular Stock Vector Images Alamy
C3h6 is an empirical formula for propene. What is the properly written empirical formula of a compound of molecular formula c6h12 with a molar mass equal to 84.16 gram. This is found by dividing the subscripts of each element by their greatest common factor, which in this case is 3. The chemical formula of propene is c3h6. To find the.
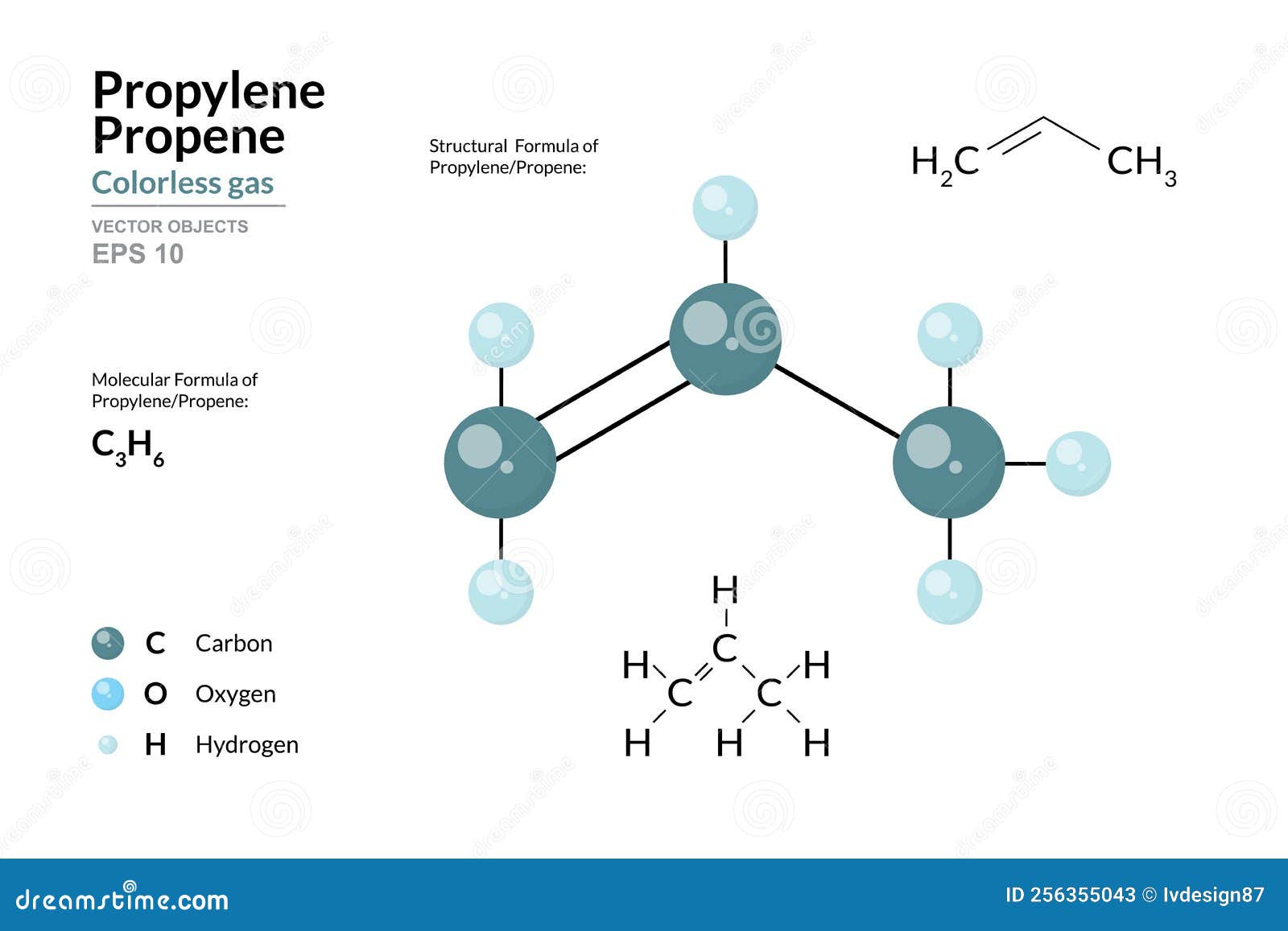
Propene, Propylene, Molecule Model, Molecular And Chemical Formula
What is the properly written empirical formula of a compound of molecular formula c6h12 with a molar mass equal to 84.16 gram. The molecular formula is the true representation of the composition of a molecule showing the number. The empirical formula for propene (c3h6) is ch2. The chemical formula of propene is c3h6. To find the percentage composition, calculate the.
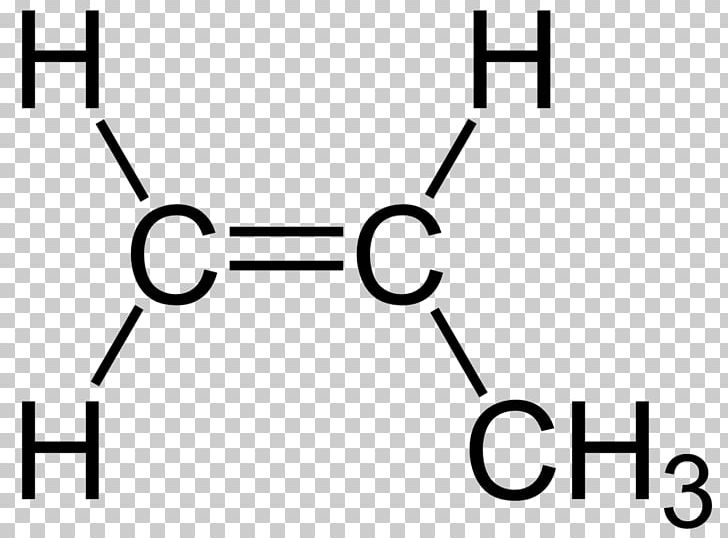
Ethylene Structural Formula Propene Chemical Bond Double Bond PNG
The chemical formula of propene is c3h6. To find the percentage composition, calculate the molar mass of each element and then divide by the molar mass of propene. C3h6 is an empirical formula for propene. This is found by dividing the subscripts of each element by their greatest common factor, which in this case is 3. What is the empirical.
This Is Found By Dividing The Subscripts Of Each Element By Their Greatest Common Factor, Which In This Case Is 3.
C3h6 is an empirical formula for propene. What is the properly written empirical formula of a compound of molecular formula c6h12 with a molar mass equal to 84.16 gram. C3h6 is an empirical formula for propene. To find the percentage composition, calculate the molar mass of each element and then divide by the molar mass of propene.
The Empirical Formula For Propene (C3H6) Is Ch2.
The chemical formula of propene is c3h6. What is the empirical formula for propene, c3h6? The molecular formula is the true representation of the composition of a molecule showing the number.