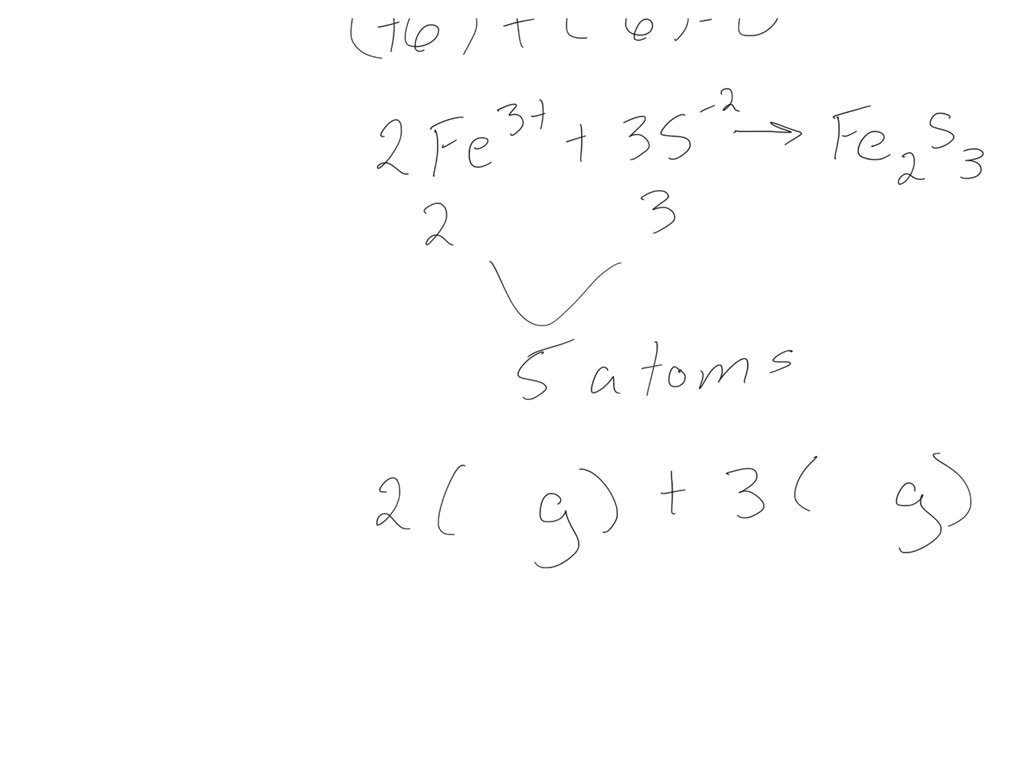What Is The Formula For The Compound Iron Iii Sulfate
What Is The Formula For The Compound Iron Iii Sulfate - Iron (iii) sulfate has a molar mass of 399.88 g/mol. 2 × ( + 3. So to neutralize both cation and anion, the total charge must be equal: The iron iii sulfate formula is written as fe2(so4)3. As iron has + 3 oxidation state (i i i), and sulphate has always − 2 charge. To find the formula for the compound iron (iii) sulfate, let's break down the components and their charges to understand how they combine. Additionally, the iron iii sulfate is composed of.
As iron has + 3 oxidation state (i i i), and sulphate has always − 2 charge. So to neutralize both cation and anion, the total charge must be equal: Additionally, the iron iii sulfate is composed of. Iron (iii) sulfate has a molar mass of 399.88 g/mol. 2 × ( + 3. The iron iii sulfate formula is written as fe2(so4)3. To find the formula for the compound iron (iii) sulfate, let's break down the components and their charges to understand how they combine.
2 × ( + 3. Additionally, the iron iii sulfate is composed of. As iron has + 3 oxidation state (i i i), and sulphate has always − 2 charge. The iron iii sulfate formula is written as fe2(so4)3. To find the formula for the compound iron (iii) sulfate, let's break down the components and their charges to understand how they combine. Iron (iii) sulfate has a molar mass of 399.88 g/mol. So to neutralize both cation and anion, the total charge must be equal:
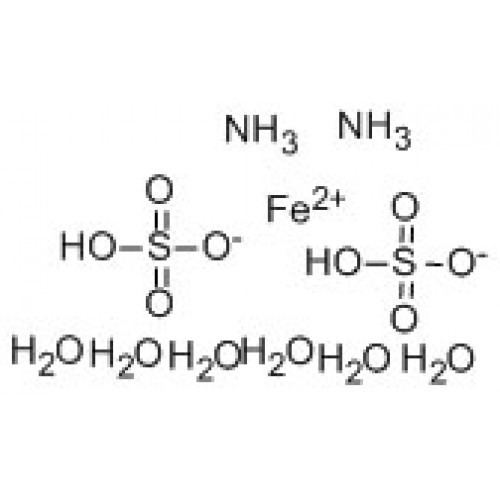
Ammonium iron(II) sulfate Alchetron, the free social encyclopedia
So to neutralize both cation and anion, the total charge must be equal: Iron (iii) sulfate has a molar mass of 399.88 g/mol. As iron has + 3 oxidation state (i i i), and sulphate has always − 2 charge. To find the formula for the compound iron (iii) sulfate, let's break down the components and their charges to understand.
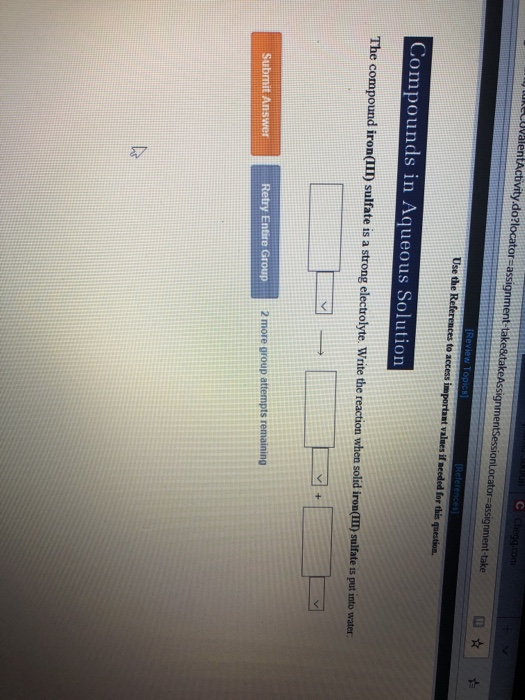
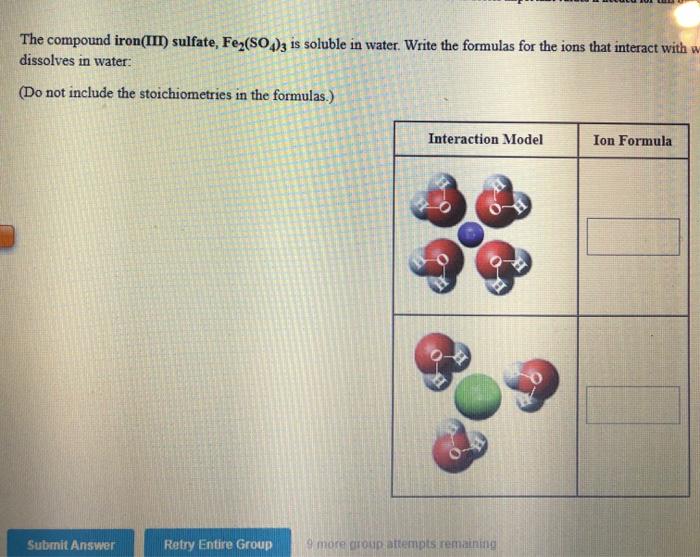
Solved Use the Compounds in Aqueous Solution do to access
To find the formula for the compound iron (iii) sulfate, let's break down the components and their charges to understand how they combine. As iron has + 3 oxidation state (i i i), and sulphate has always − 2 charge. Iron (iii) sulfate has a molar mass of 399.88 g/mol. Additionally, the iron iii sulfate is composed of. So to.
Solved Complete the following for the compound iron(III)
So to neutralize both cation and anion, the total charge must be equal: As iron has + 3 oxidation state (i i i), and sulphate has always − 2 charge. To find the formula for the compound iron (iii) sulfate, let's break down the components and their charges to understand how they combine. 2 × ( + 3. Additionally, the.
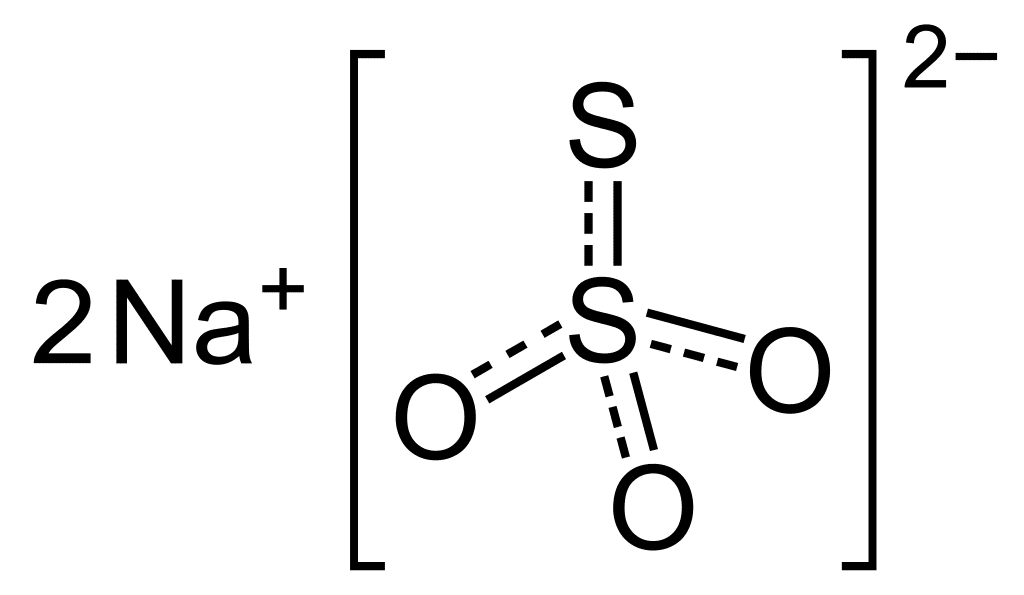
Sodium Thiosulfate for Hatcheries and Aquaculture Facilities Syndel
As iron has + 3 oxidation state (i i i), and sulphate has always − 2 charge. Additionally, the iron iii sulfate is composed of. So to neutralize both cation and anion, the total charge must be equal: To find the formula for the compound iron (iii) sulfate, let's break down the components and their charges to understand how they.
Examples of Iron Compounds Iron IronOxide IronSulfate
So to neutralize both cation and anion, the total charge must be equal: To find the formula for the compound iron (iii) sulfate, let's break down the components and their charges to understand how they combine. Additionally, the iron iii sulfate is composed of. The iron iii sulfate formula is written as fe2(so4)3. Iron (iii) sulfate has a molar mass.
Ammonium iron(II) sulfate Alchetron, the free social encyclopedia
The iron iii sulfate formula is written as fe2(so4)3. To find the formula for the compound iron (iii) sulfate, let's break down the components and their charges to understand how they combine. Iron (iii) sulfate has a molar mass of 399.88 g/mol. Additionally, the iron iii sulfate is composed of. So to neutralize both cation and anion, the total charge.
SOLVED For the compound iron (III) sulfide, answer the following
So to neutralize both cation and anion, the total charge must be equal: 2 × ( + 3. Additionally, the iron iii sulfate is composed of. The iron iii sulfate formula is written as fe2(so4)3. As iron has + 3 oxidation state (i i i), and sulphate has always − 2 charge.
Solved The compound iron(III) sulfate, Fe2(SO4)3 is soluble
To find the formula for the compound iron (iii) sulfate, let's break down the components and their charges to understand how they combine. Iron (iii) sulfate has a molar mass of 399.88 g/mol. The iron iii sulfate formula is written as fe2(so4)3. 2 × ( + 3. As iron has + 3 oxidation state (i i i), and sulphate has.
Solved Use the Compounds in Aqueous Solution do to access
2 × ( + 3. To find the formula for the compound iron (iii) sulfate, let's break down the components and their charges to understand how they combine. As iron has + 3 oxidation state (i i i), and sulphate has always − 2 charge. So to neutralize both cation and anion, the total charge must be equal: Additionally, the.
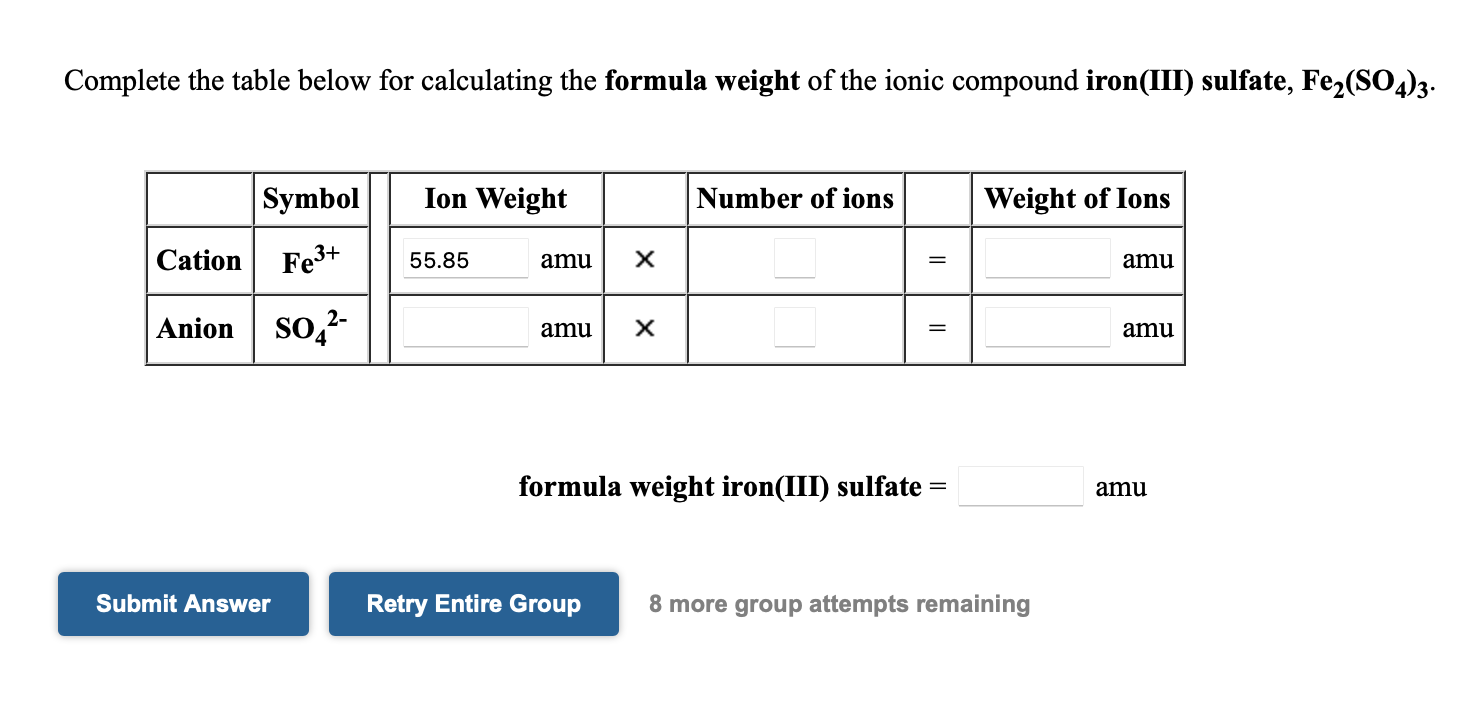
Solved Complete the table below for calculating the formula
As iron has + 3 oxidation state (i i i), and sulphate has always − 2 charge. 2 × ( + 3. The iron iii sulfate formula is written as fe2(so4)3. Iron (iii) sulfate has a molar mass of 399.88 g/mol. To find the formula for the compound iron (iii) sulfate, let's break down the components and their charges to.
To Find The Formula For The Compound Iron (Iii) Sulfate, Let's Break Down The Components And Their Charges To Understand How They Combine.
Iron (iii) sulfate has a molar mass of 399.88 g/mol. The iron iii sulfate formula is written as fe2(so4)3. So to neutralize both cation and anion, the total charge must be equal: Additionally, the iron iii sulfate is composed of.
As Iron Has + 3 Oxidation State (I I I), And Sulphate Has Always − 2 Charge.
2 × ( + 3.