What Is The Hybridization Of The Central Atom In Sf4
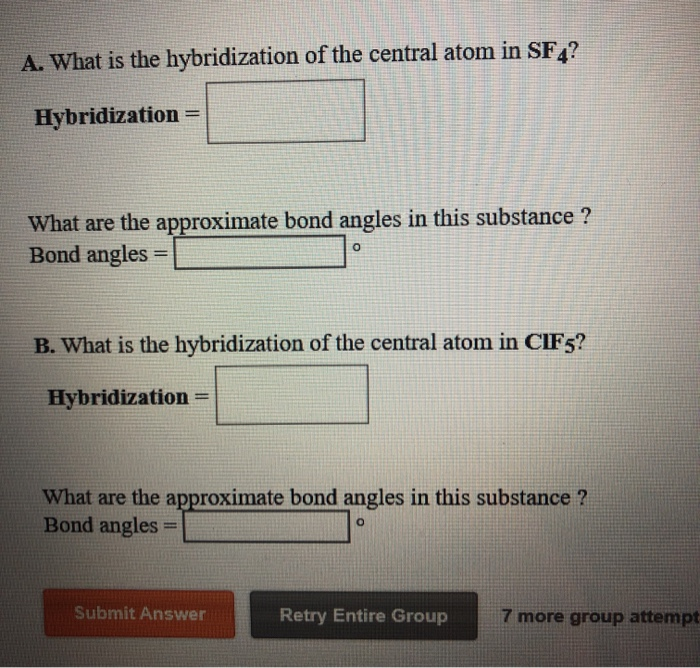
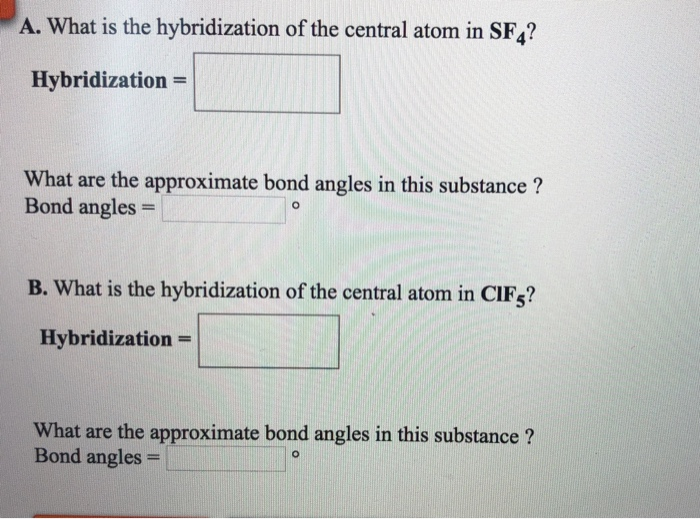
What Is The Hybridization Of The Central Atom In Sf4 - Sf₄ has only one lone pair and four sigma bonds of f. Thus, the total electron density regions of sulphur atom are 5. What is the hybridization of sf₄? So, to explain in simple terms, its. The central atom is s. One pair of electrons or a lone pair is present on the sulphur atom. In the sulfur tetrafluoride (sf4) molecule, the central sulfur atom is surrounded by four fluorine atoms and one lone pair of electrons. The hybridization of sulfur in sf4 is sp3d. The bond angles in sf4 are approximately 120° and 90°. The central atom in sf4 is sulfur (s).
One pair of electrons or a lone pair is present on the sulphur atom. In the sulfur tetrafluoride (sf4) molecule, the central sulfur atom is surrounded by four fluorine atoms and one lone pair of electrons. The hybridization of sulfur in sf4 is sp3d. The central atom is s. The central atom in sf4 is sulfur (s). The central atom in sf4 is sulfur (s). The bond angles in sf4 are approximately 120° and 90°. What is the hybridization of sf₄? Thus, the total electron density regions of sulphur atom are 5. Sf₄ has only one lone pair and four sigma bonds of f.
So, to explain in simple terms, its. Thus, the total electron density regions of sulphur atom are 5. The central atom is s. The central atom in sf4 is sulfur (s). The hybridization of sulfur in sf4 is sp3d. In the sulfur tetrafluoride (sf4) molecule, the central sulfur atom is surrounded by four fluorine atoms and one lone pair of electrons. What is the hybridization of sf₄? One pair of electrons or a lone pair is present on the sulphur atom. The central atom in sf4 is sulfur (s). The bond angles in sf4 are approximately 120° and 90°.
Solved A. What is the hybridization of the central atom in
Thus, the total electron density regions of sulphur atom are 5. What is the hybridization of sf₄? Sf₄ has only one lone pair and four sigma bonds of f. In the sulfur tetrafluoride (sf4) molecule, the central sulfur atom is surrounded by four fluorine atoms and one lone pair of electrons. The central atom is s.
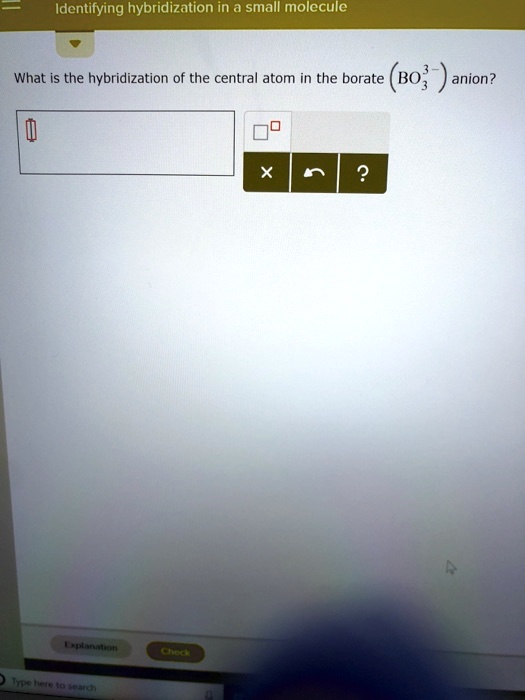
SOLVED Identifying hybridization in small molecule What is the
The central atom is s. One pair of electrons or a lone pair is present on the sulphur atom. Thus, the total electron density regions of sulphur atom are 5. Sf₄ has only one lone pair and four sigma bonds of f. The central atom in sf4 is sulfur (s).
Solved A. What is the hybridization of the central atom in
One pair of electrons or a lone pair is present on the sulphur atom. In the sulfur tetrafluoride (sf4) molecule, the central sulfur atom is surrounded by four fluorine atoms and one lone pair of electrons. So, to explain in simple terms, its. The bond angles in sf4 are approximately 120° and 90°. The central atom is s.
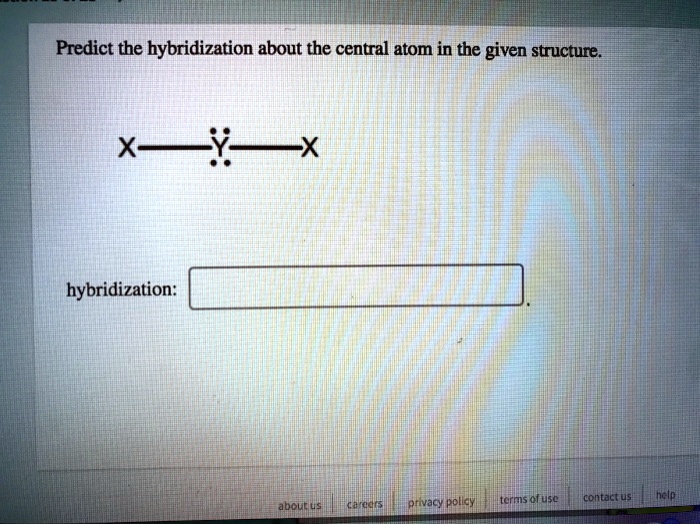
[ANSWERED] A. What is the hybridization of the central atom in IF5
One pair of electrons or a lone pair is present on the sulphur atom. Sf₄ has only one lone pair and four sigma bonds of f. Thus, the total electron density regions of sulphur atom are 5. The bond angles in sf4 are approximately 120° and 90°. The central atom in sf4 is sulfur (s).
Solved A. What is the hybridization of the central atom in
The central atom is s. In the sulfur tetrafluoride (sf4) molecule, the central sulfur atom is surrounded by four fluorine atoms and one lone pair of electrons. The central atom in sf4 is sulfur (s). One pair of electrons or a lone pair is present on the sulphur atom. The bond angles in sf4 are approximately 120° and 90°.
Solved What is the hybridization of the central atom in
The central atom in sf4 is sulfur (s). Thus, the total electron density regions of sulphur atom are 5. What is the hybridization of sf₄? The bond angles in sf4 are approximately 120° and 90°. One pair of electrons or a lone pair is present on the sulphur atom.
Solved A. What is the hybridization of the central atom in
The central atom in sf4 is sulfur (s). So, to explain in simple terms, its. The central atom in sf4 is sulfur (s). The bond angles in sf4 are approximately 120° and 90°. In the sulfur tetrafluoride (sf4) molecule, the central sulfur atom is surrounded by four fluorine atoms and one lone pair of electrons.
Solved A. What is the hybridization of the central atom in
Sf₄ has only one lone pair and four sigma bonds of f. The bond angles in sf4 are approximately 120° and 90°. What is the hybridization of sf₄? The central atom is s. So, to explain in simple terms, its.
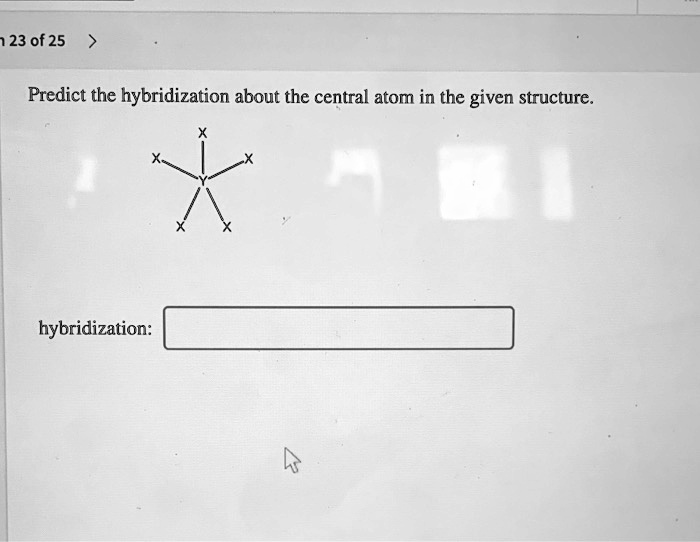
SOLVED 123 of 25 Predict the hybridization about the central atom in
The hybridization of sulfur in sf4 is sp3d. The bond angles in sf4 are approximately 120° and 90°. What is the hybridization of sf₄? The central atom is s. The central atom in sf4 is sulfur (s).
The Central Atom Is S.
The central atom in sf4 is sulfur (s). What is the hybridization of sf₄? The hybridization of sulfur in sf4 is sp3d. One pair of electrons or a lone pair is present on the sulphur atom.
The Bond Angles In Sf4 Are Approximately 120° And 90°.
Thus, the total electron density regions of sulphur atom are 5. The central atom in sf4 is sulfur (s). In the sulfur tetrafluoride (sf4) molecule, the central sulfur atom is surrounded by four fluorine atoms and one lone pair of electrons. So, to explain in simple terms, its.
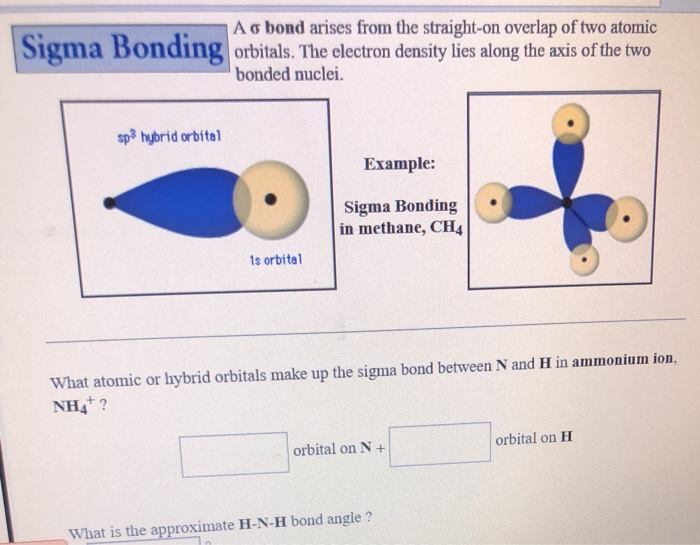

![[ANSWERED] A. What is the hybridization of the central atom in IF5](https://media.kunduz.com/media/sug-question/raw/52262186-1659250506.6599846.jpeg?h=512)