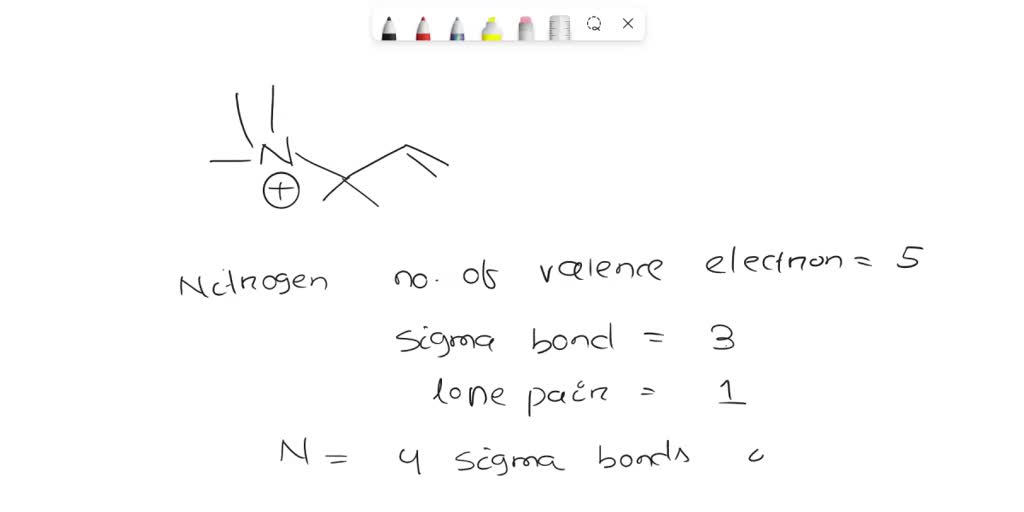
What Is The Hybridization Of The Nitrogen Atoms In N2
What Is The Hybridization Of The Nitrogen Atoms In N2 - The hybridization of nitrogen in n 2 is sp. This is because each nitrogen atom is bonded to one other nitrogen atom and there are two lone. The nitrogen n2 molecule has a triple bond between the two nitrogen atoms.this means here are two pi bonds, and that means. Today in this video we will help you determine the hybridization of nitrogen, having the chemical formula of n2. The n2 molecule does not undergo hybridization as it consists of two nitrogen atoms bonded together by a triple bond. Nitrogen gas is shown below. When determining hybridization, you must count. It has a triple bond and one lone pair on each nitrogen atom.
This is because each nitrogen atom is bonded to one other nitrogen atom and there are two lone. The hybridization of nitrogen in n 2 is sp. When determining hybridization, you must count. The n2 molecule does not undergo hybridization as it consists of two nitrogen atoms bonded together by a triple bond. Nitrogen gas is shown below. It has a triple bond and one lone pair on each nitrogen atom. Today in this video we will help you determine the hybridization of nitrogen, having the chemical formula of n2. The nitrogen n2 molecule has a triple bond between the two nitrogen atoms.this means here are two pi bonds, and that means.
This is because each nitrogen atom is bonded to one other nitrogen atom and there are two lone. Nitrogen gas is shown below. Today in this video we will help you determine the hybridization of nitrogen, having the chemical formula of n2. The hybridization of nitrogen in n 2 is sp. The nitrogen n2 molecule has a triple bond between the two nitrogen atoms.this means here are two pi bonds, and that means. The n2 molecule does not undergo hybridization as it consists of two nitrogen atoms bonded together by a triple bond. When determining hybridization, you must count. It has a triple bond and one lone pair on each nitrogen atom.
Solved Question 52 What Is The Hybridization Of The Indic...
The hybridization of nitrogen in n 2 is sp. Nitrogen gas is shown below. The n2 molecule does not undergo hybridization as it consists of two nitrogen atoms bonded together by a triple bond. When determining hybridization, you must count. This is because each nitrogen atom is bonded to one other nitrogen atom and there are two lone.
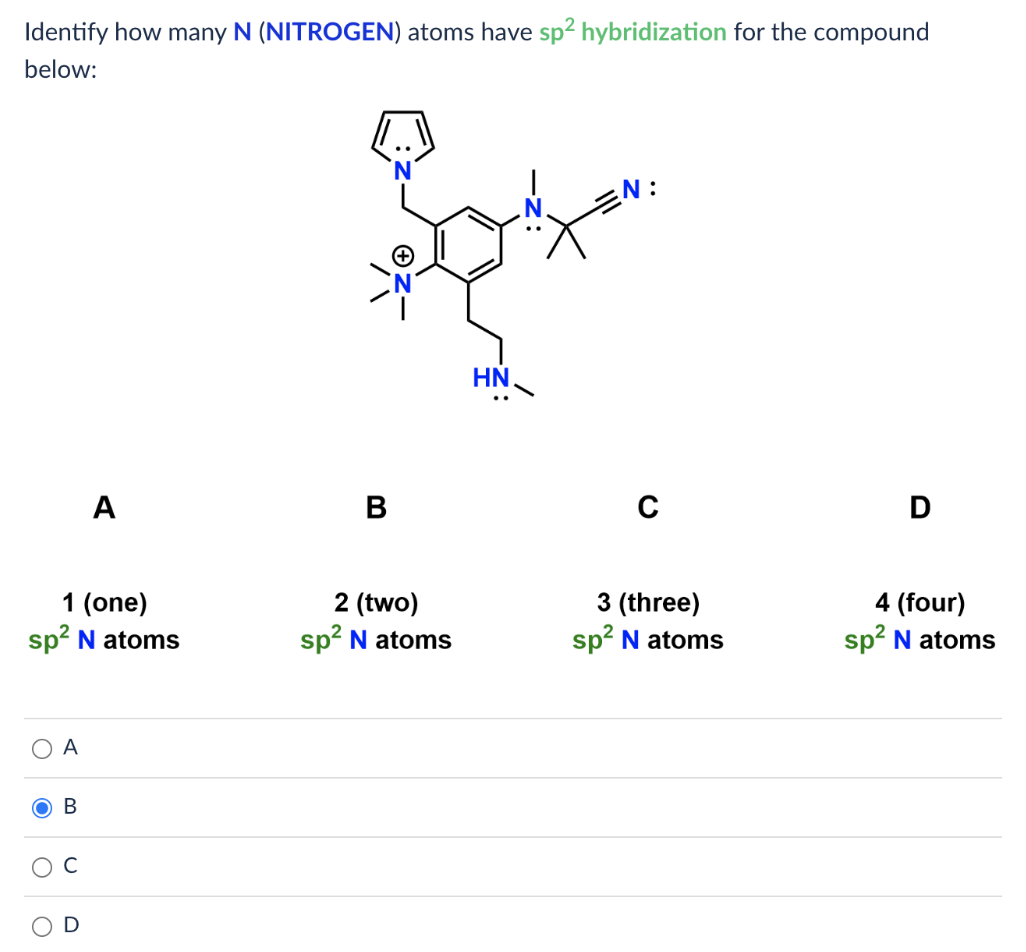
[Solved] Identify how many N (NITROGEN) atoms have sp hybridization for
Today in this video we will help you determine the hybridization of nitrogen, having the chemical formula of n2. It has a triple bond and one lone pair on each nitrogen atom. This is because each nitrogen atom is bonded to one other nitrogen atom and there are two lone. When determining hybridization, you must count. The n2 molecule does.
[Solved] Identify how many N (NITROGEN) atoms have sp2
Today in this video we will help you determine the hybridization of nitrogen, having the chemical formula of n2. This is because each nitrogen atom is bonded to one other nitrogen atom and there are two lone. When determining hybridization, you must count. Nitrogen gas is shown below. The n2 molecule does not undergo hybridization as it consists of two.
[Solved] Identify how many N ( NITROGEN ) atoms have sp 3 hybridization
Nitrogen gas is shown below. This is because each nitrogen atom is bonded to one other nitrogen atom and there are two lone. The nitrogen n2 molecule has a triple bond between the two nitrogen atoms.this means here are two pi bonds, and that means. Today in this video we will help you determine the hybridization of nitrogen, having the.
Solved Identify how many N (NITROGEN) atoms have \\(
The nitrogen n2 molecule has a triple bond between the two nitrogen atoms.this means here are two pi bonds, and that means. The hybridization of nitrogen in n 2 is sp. Nitrogen gas is shown below. This is because each nitrogen atom is bonded to one other nitrogen atom and there are two lone. Today in this video we will.
[Solved] Identify how many N (NITROGEN) atoms have sp hybridization for
The n2 molecule does not undergo hybridization as it consists of two nitrogen atoms bonded together by a triple bond. Today in this video we will help you determine the hybridization of nitrogen, having the chemical formula of n2. This is because each nitrogen atom is bonded to one other nitrogen atom and there are two lone. When determining hybridization,.
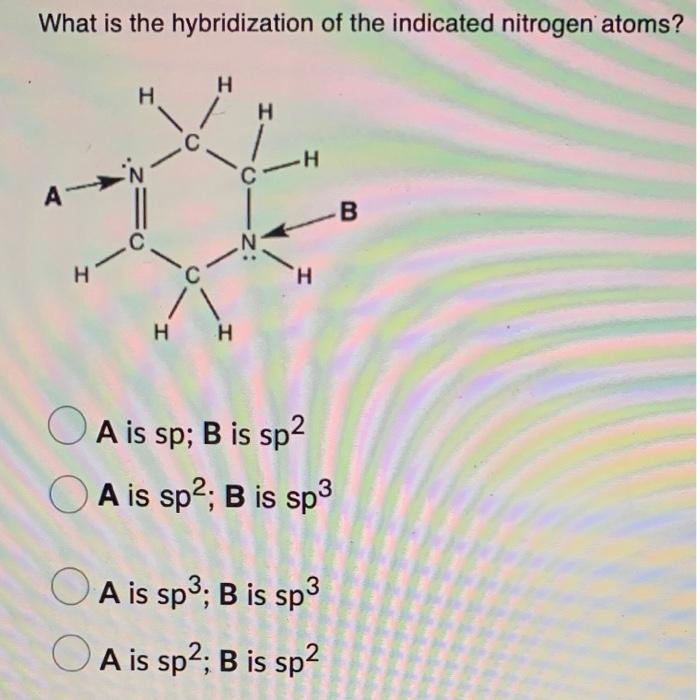
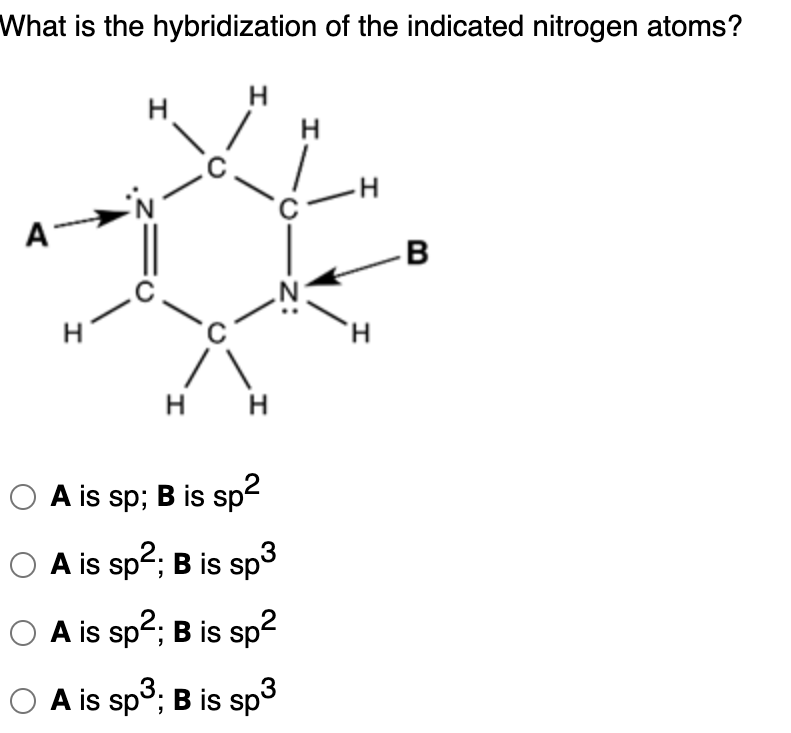
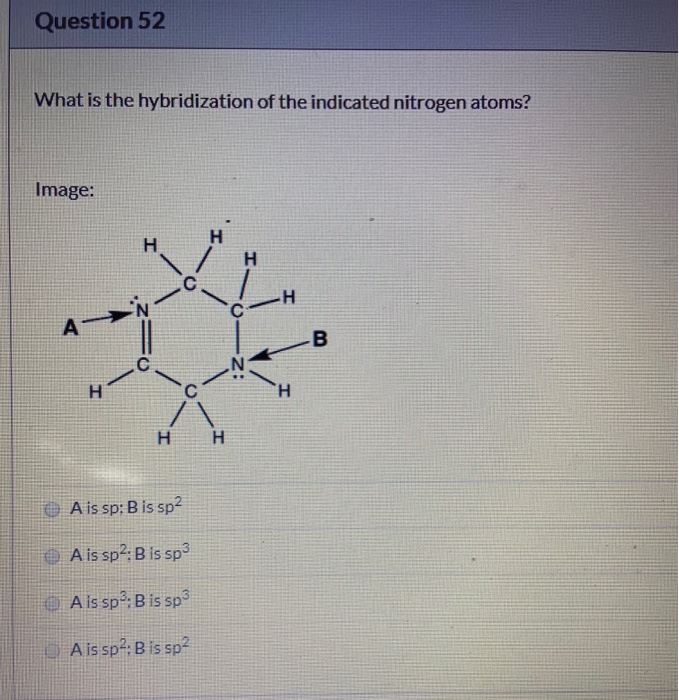
Solved What is the hybridization of the indicated nitrogen
The nitrogen n2 molecule has a triple bond between the two nitrogen atoms.this means here are two pi bonds, and that means. When determining hybridization, you must count. The n2 molecule does not undergo hybridization as it consists of two nitrogen atoms bonded together by a triple bond. This is because each nitrogen atom is bonded to one other nitrogen.
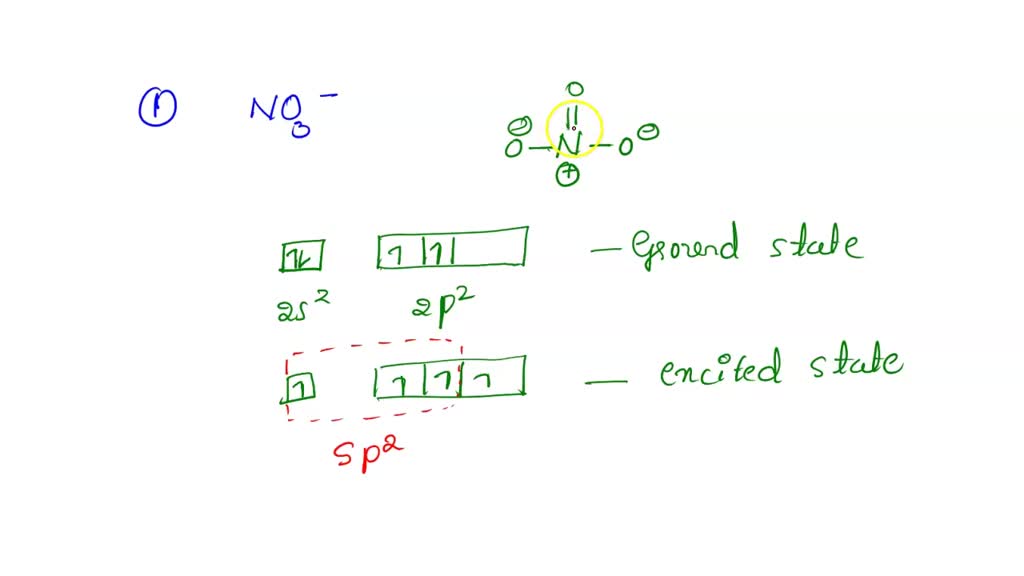
SOLVED What is the hybridization of nitrogen in the nitrite ion
This is because each nitrogen atom is bonded to one other nitrogen atom and there are two lone. The hybridization of nitrogen in n 2 is sp. Today in this video we will help you determine the hybridization of nitrogen, having the chemical formula of n2. When determining hybridization, you must count. Nitrogen gas is shown below.
Solved What is the hybridization of the indicated nitrogen
Today in this video we will help you determine the hybridization of nitrogen, having the chemical formula of n2. The hybridization of nitrogen in n 2 is sp. Nitrogen gas is shown below. This is because each nitrogen atom is bonded to one other nitrogen atom and there are two lone. When determining hybridization, you must count.
SOLVED Please identify the type of hybridization (sp, sp2, sp3) for
This is because each nitrogen atom is bonded to one other nitrogen atom and there are two lone. Nitrogen gas is shown below. When determining hybridization, you must count. The n2 molecule does not undergo hybridization as it consists of two nitrogen atoms bonded together by a triple bond. Today in this video we will help you determine the hybridization.
It Has A Triple Bond And One Lone Pair On Each Nitrogen Atom.
The nitrogen n2 molecule has a triple bond between the two nitrogen atoms.this means here are two pi bonds, and that means. This is because each nitrogen atom is bonded to one other nitrogen atom and there are two lone. The hybridization of nitrogen in n 2 is sp. The n2 molecule does not undergo hybridization as it consists of two nitrogen atoms bonded together by a triple bond.
When Determining Hybridization, You Must Count.
Today in this video we will help you determine the hybridization of nitrogen, having the chemical formula of n2. Nitrogen gas is shown below.
![[Solved] Identify how many N (NITROGEN) atoms have sp2](https://media.cheggcdn.com/media/250/25001471-53e2-40e1-97ef-668977575fc1/phppbkvdZ)