What Is The Molecular Geometry Of Seh2
What Is The Molecular Geometry Of Seh2 - Molecular geometry is the shape of the molecule. The lewis structure for se is se::, for h is h. Selenium has 6 valence electrons. This is because selenium (se) has two bonded pairs of electrons (from the two hydrogen. There are 3 steps to solve this one. The lewis diagram for seh2 is: In seh2, 2 electrons are used for bonding with 2 hydrogen atoms, leaving 4. Valence shell electron pair repulsion t. Selenium arranges itself with hydrogen atoms. The lewis diagram for seh2 is:
There are 3 steps to solve this one. Valence shell electron pair repulsion t. Molecular geometry is the shape of the molecule. The shape of seh2 (selenium hydride) can be determined using the valence shell electron pair repulsion (vsepr) model. The lewis diagram for seh2 is: Selenium arranges itself with hydrogen atoms. The lewis structure for se is se::, for h is h. The lewis diagram for seh2 is: Selenium has 6 valence electrons. What is the molecular geometry of seh2 chegg?
Selenium has 6 valence electrons. This is because selenium (se) has two bonded pairs of electrons (from the two hydrogen. What is the molecular geometry of seh2 chegg? Selenium arranges itself with hydrogen atoms. Molecular geometry is the shape of the molecule. There are 3 steps to solve this one. The lewis diagram for seh2 is: Valence shell electron pair repulsion t. In seh2, 2 electrons are used for bonding with 2 hydrogen atoms, leaving 4. The lewis structure for se is se::, for h is h.
Best Molecular Geometry And Vsepr Theory PNG GM
In seh2, 2 electrons are used for bonding with 2 hydrogen atoms, leaving 4. Valence shell electron pair repulsion t. Molecular geometry is the shape of the molecule. The lewis diagram for seh2 is: The lewis structure for se is se::, for h is h.
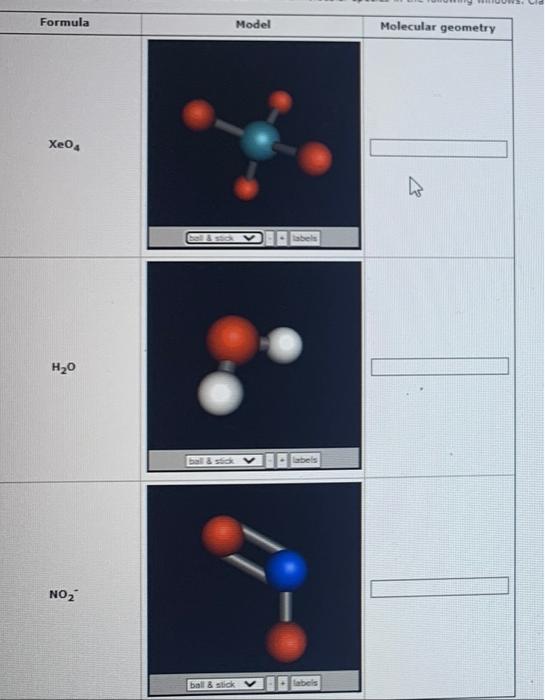
Solved Formula Model Molecular geometry SeH2 ball & stick
Selenium arranges itself with hydrogen atoms. This is because selenium (se) has two bonded pairs of electrons (from the two hydrogen. What is the molecular geometry of seh2 chegg? The lewis diagram for seh2 is: Molecular geometry is the shape of the molecule.
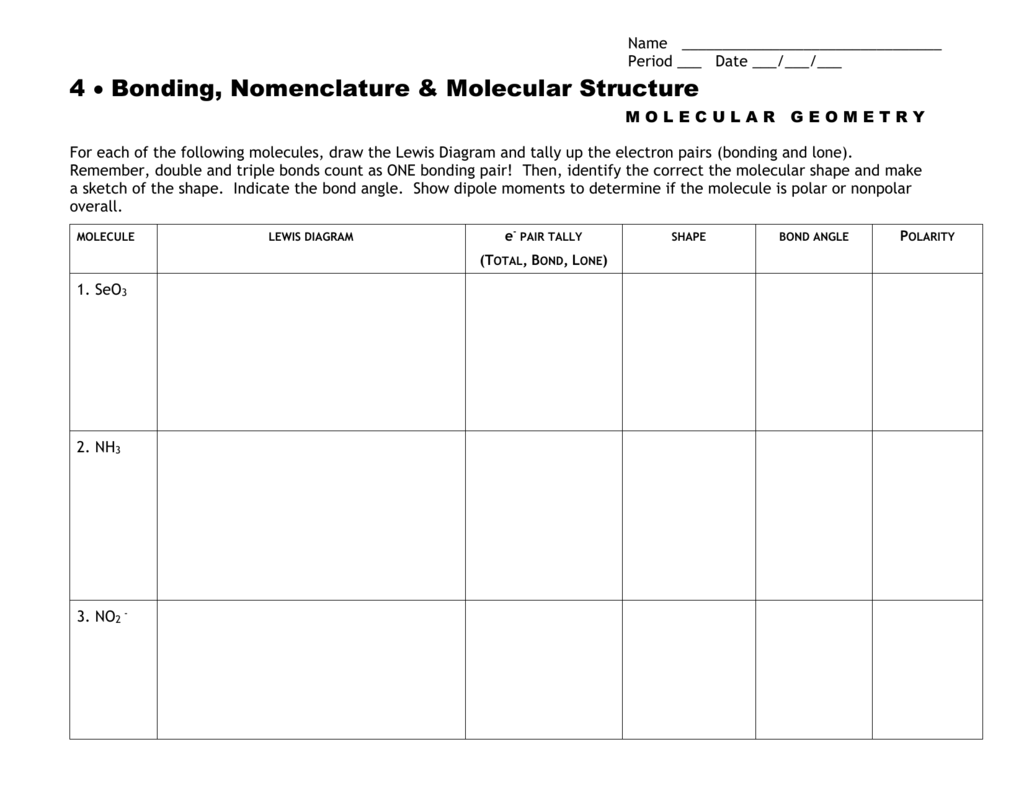
molecular geometry worksheet
Molecular geometry is the shape of the molecule. What is the molecular geometry of seh2 chegg? The lewis diagram for seh2 is: The central atom in seh2 is selenium (se). The lewis structure for se is se::, for h is h.
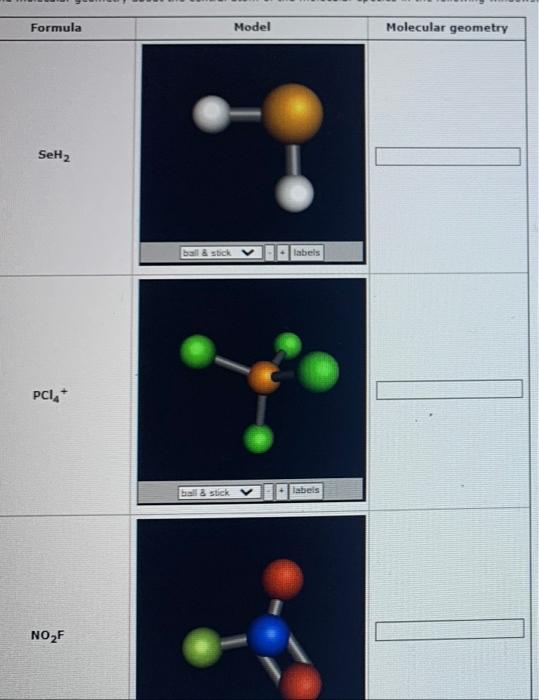
SOLVED For each formula given, draw the Lewis diagram, determine the
Valence shell electron pair repulsion t. The lewis structure for se is se::, for h is h. The shape of seh2 (selenium hydride) can be determined using the valence shell electron pair repulsion (vsepr) model. Molecular geometry is the shape of the molecule. The central atom in seh2 is selenium (se).
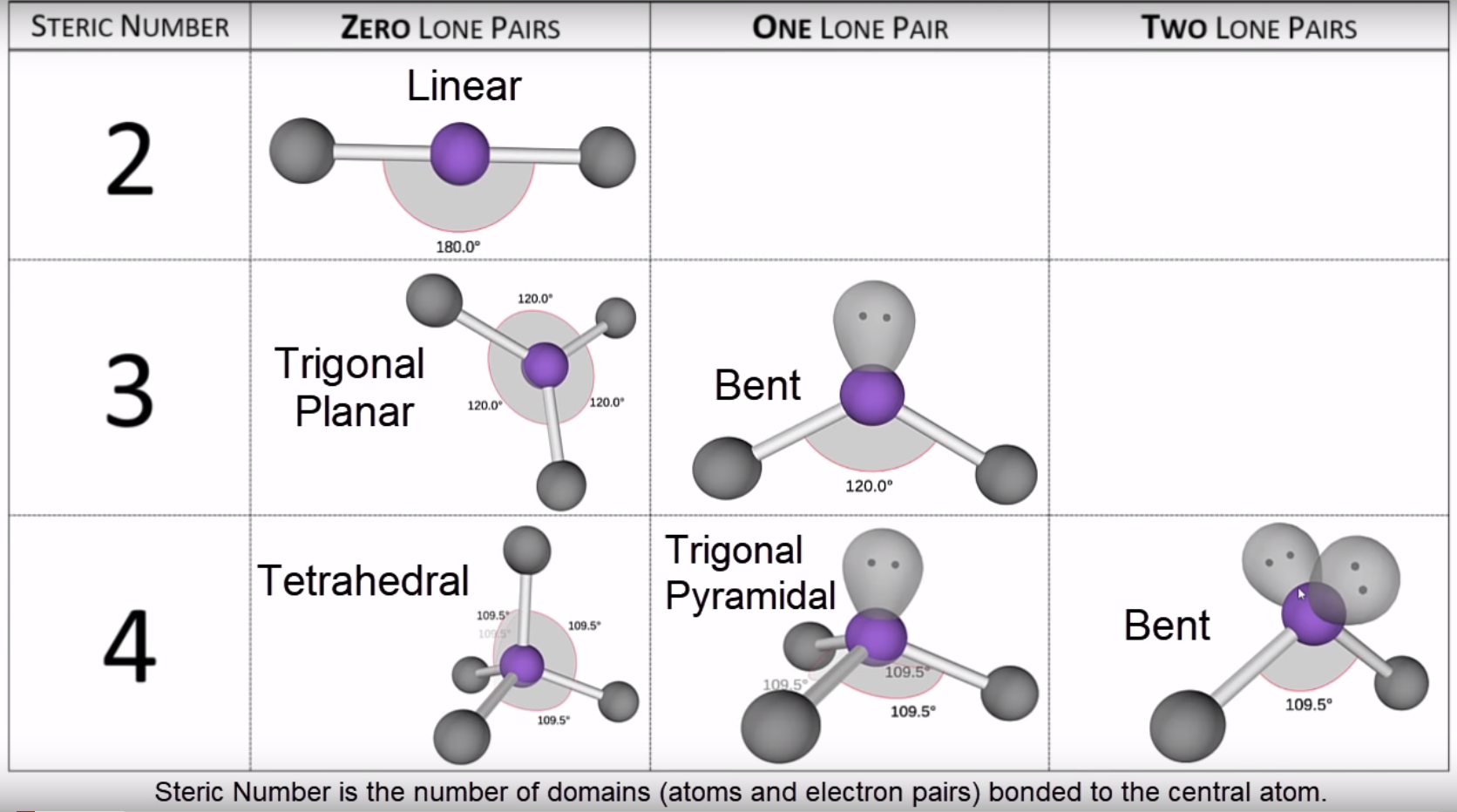
Molecular Geometry (Shape)
The shape of seh2 (selenium hydride) can be determined using the valence shell electron pair repulsion (vsepr) model. Molecular geometry is the shape of the molecule. The lewis structure for se is se::, for h is h. There are 3 steps to solve this one. This is because selenium (se) has two bonded pairs of electrons (from the two hydrogen.
Chapter 6.3 VSEPR Molecular Geometry Chemistry LibreTexts
In seh2, 2 electrons are used for bonding with 2 hydrogen atoms, leaving 4. The shape of seh2 (selenium hydride) can be determined using the valence shell electron pair repulsion (vsepr) model. The lewis structure for se is se::, for h is h. Selenium arranges itself with hydrogen atoms. Valence shell electron pair repulsion t.
Molecular Geometry Worksheet Answer Key Naturalium
The lewis diagram for seh2 is: This is because selenium (se) has two bonded pairs of electrons (from the two hydrogen. The lewis diagram for seh2 is: Selenium has 6 valence electrons. The central atom in seh2 is selenium (se).
Solved Formula Model Molecular geometry SeH2 ball & stick
The central atom in seh2 is selenium (se). Valence shell electron pair repulsion t. Molecular geometry is the shape of the molecule. The shape of seh2 (selenium hydride) can be determined using the valence shell electron pair repulsion (vsepr) model. The lewis diagram for seh2 is:
Molecular Geometry Worksheet
The lewis diagram for seh2 is: There are 3 steps to solve this one. Selenium has 6 valence electrons. This is because selenium (se) has two bonded pairs of electrons (from the two hydrogen. The lewis diagram for seh2 is:
SOLVED For which of the following molecules will the electronpair
There are 3 steps to solve this one. The lewis structure for se is se::, for h is h. In seh2, 2 electrons are used for bonding with 2 hydrogen atoms, leaving 4. The lewis diagram for seh2 is: The shape of seh2 (selenium hydride) can be determined using the valence shell electron pair repulsion (vsepr) model.
The Lewis Structure For Se Is Se::, For H Is H.
Selenium arranges itself with hydrogen atoms. Selenium has 6 valence electrons. Valence shell electron pair repulsion t. The lewis diagram for seh2 is:
The Central Atom In Seh2 Is Selenium (Se).
This is because selenium (se) has two bonded pairs of electrons (from the two hydrogen. There are 3 steps to solve this one. The lewis diagram for seh2 is: Molecular geometry is the shape of the molecule.
In Seh2, 2 Electrons Are Used For Bonding With 2 Hydrogen Atoms, Leaving 4.
What is the molecular geometry of seh2 chegg? The shape of seh2 (selenium hydride) can be determined using the valence shell electron pair repulsion (vsepr) model.