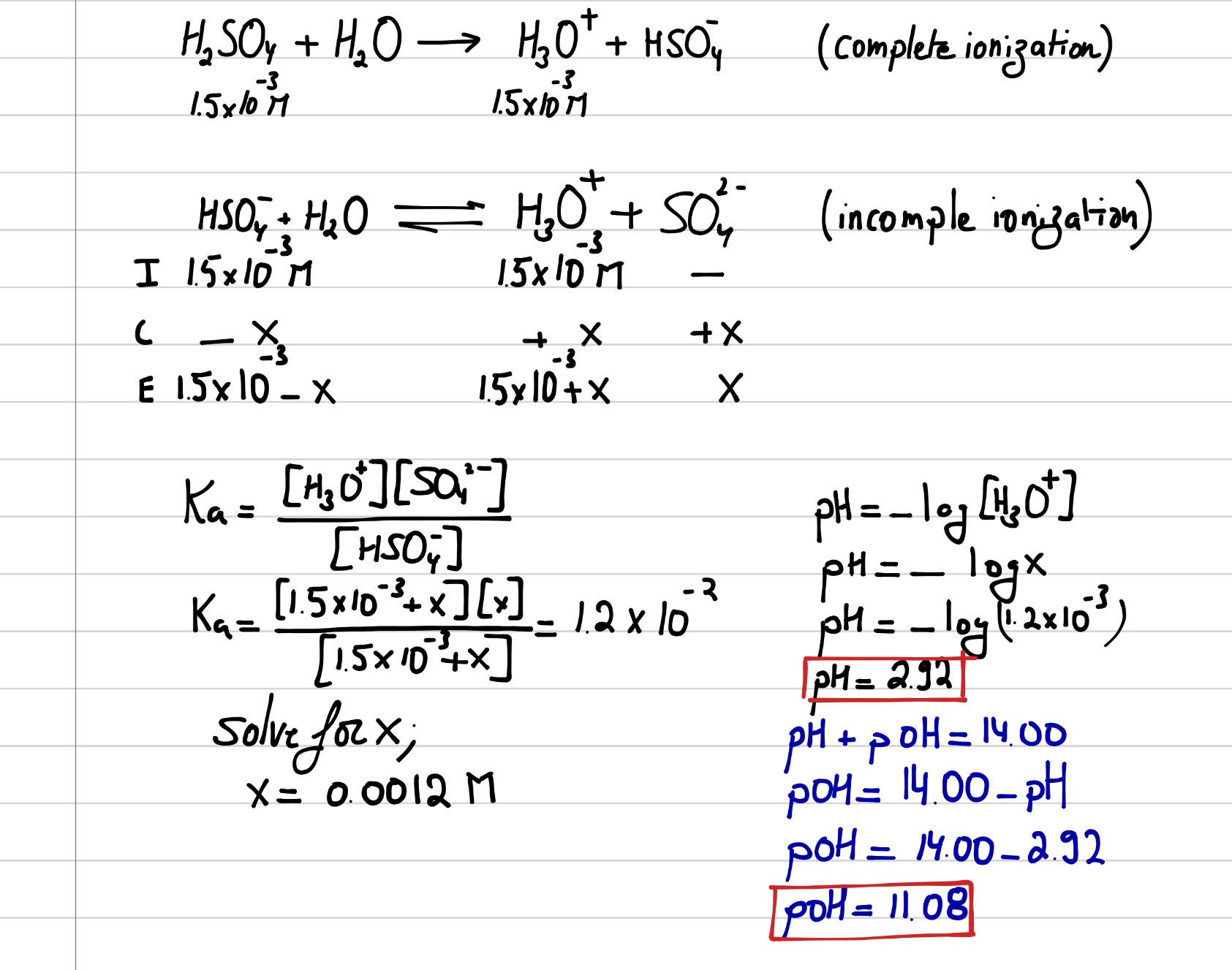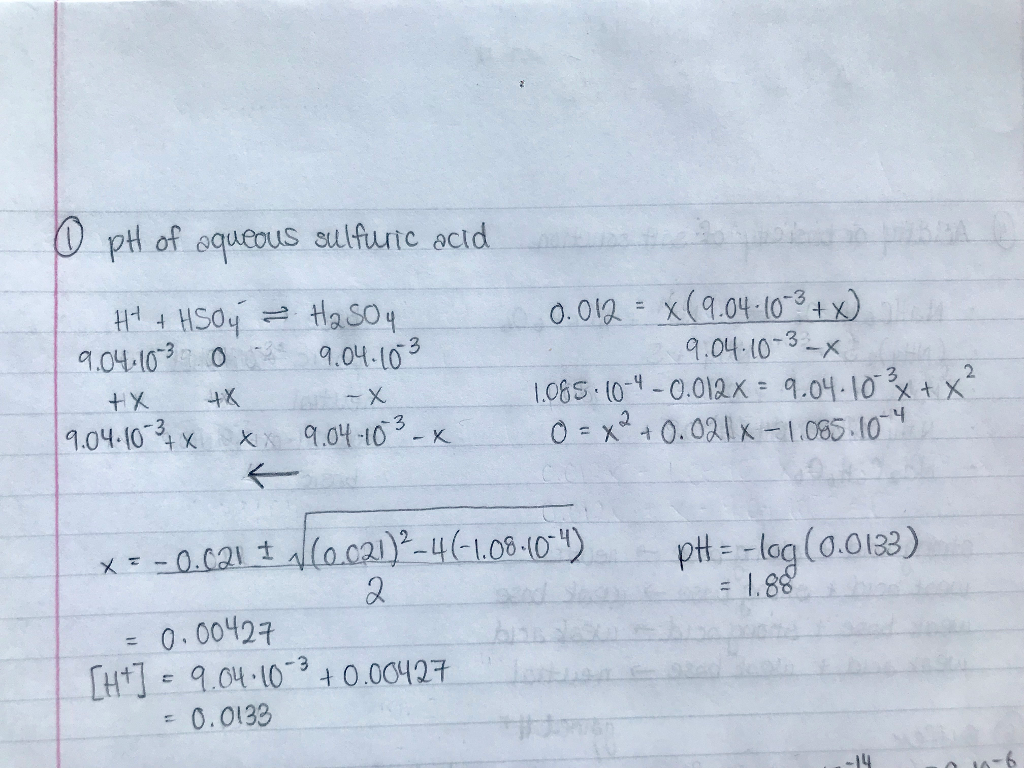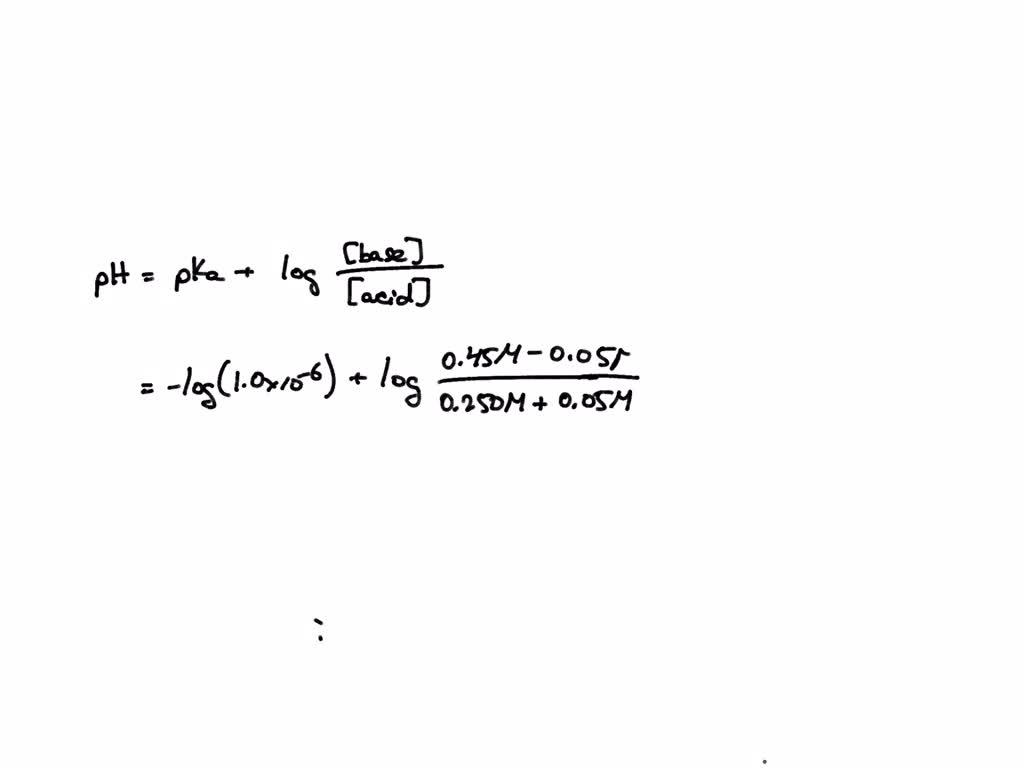What Is The Ph Of 0 45 M Of H2So4
What Is The Ph Of 0 45 M Of H2So4 - Thus [ h 3 o +] = 2 ×. The correct ph for a 0.45m solution of h2so4 is 0.95. The ph calculator can determine the ph from h⁺ molar concentration, or ka, and the concentration of a solution. What is the ph of $ 0.45m $ $ {h_2}s{o_4} $ ?. The ph of a 0.45m solution of h₂so₄ is calculated by first determining the concentration of h⁺ in the solution, then applying the ph formula. Sulphuric acid is reasonably treated as a strong diprotic acid that gives stoichiometric [h 3o +] and [so 42−] on dissolution. Matching this value to the choices provided, the closest option is d. Sulphuric acid is reasonably treated as a strong diprotic acid that gives stoichiometric [ h 3 o +] and [ s o 4 2 −] on dissolution. Ph of a solution is the negative logarithm of concentration of $ {h^ + }\\;
Sulphuric acid is reasonably treated as a strong diprotic acid that gives stoichiometric [h 3o +] and [so 42−] on dissolution. Thus [ h 3 o +] = 2 ×. What is the ph of $ 0.45m $ $ {h_2}s{o_4} $ ?. Ph of a solution is the negative logarithm of concentration of $ {h^ + }\\; The correct ph for a 0.45m solution of h2so4 is 0.95. Sulphuric acid is reasonably treated as a strong diprotic acid that gives stoichiometric [ h 3 o +] and [ s o 4 2 −] on dissolution. The ph of a 0.45m solution of h₂so₄ is calculated by first determining the concentration of h⁺ in the solution, then applying the ph formula. The ph calculator can determine the ph from h⁺ molar concentration, or ka, and the concentration of a solution. Matching this value to the choices provided, the closest option is d.
Matching this value to the choices provided, the closest option is d. The correct ph for a 0.45m solution of h2so4 is 0.95. Thus [ h 3 o +] = 2 ×. Sulphuric acid is reasonably treated as a strong diprotic acid that gives stoichiometric [ h 3 o +] and [ s o 4 2 −] on dissolution. What is the ph of $ 0.45m $ $ {h_2}s{o_4} $ ?. The ph of a 0.45m solution of h₂so₄ is calculated by first determining the concentration of h⁺ in the solution, then applying the ph formula. Sulphuric acid is reasonably treated as a strong diprotic acid that gives stoichiometric [h 3o +] and [so 42−] on dissolution. Ph of a solution is the negative logarithm of concentration of $ {h^ + }\\; The ph calculator can determine the ph from h⁺ molar concentration, or ka, and the concentration of a solution.
calculate the pH of 0.05 H2SO4 solution
The ph calculator can determine the ph from h⁺ molar concentration, or ka, and the concentration of a solution. What is the ph of $ 0.45m $ $ {h_2}s{o_4} $ ?. Sulphuric acid is reasonably treated as a strong diprotic acid that gives stoichiometric [ h 3 o +] and [ s o 4 2 −] on dissolution. Sulphuric acid.
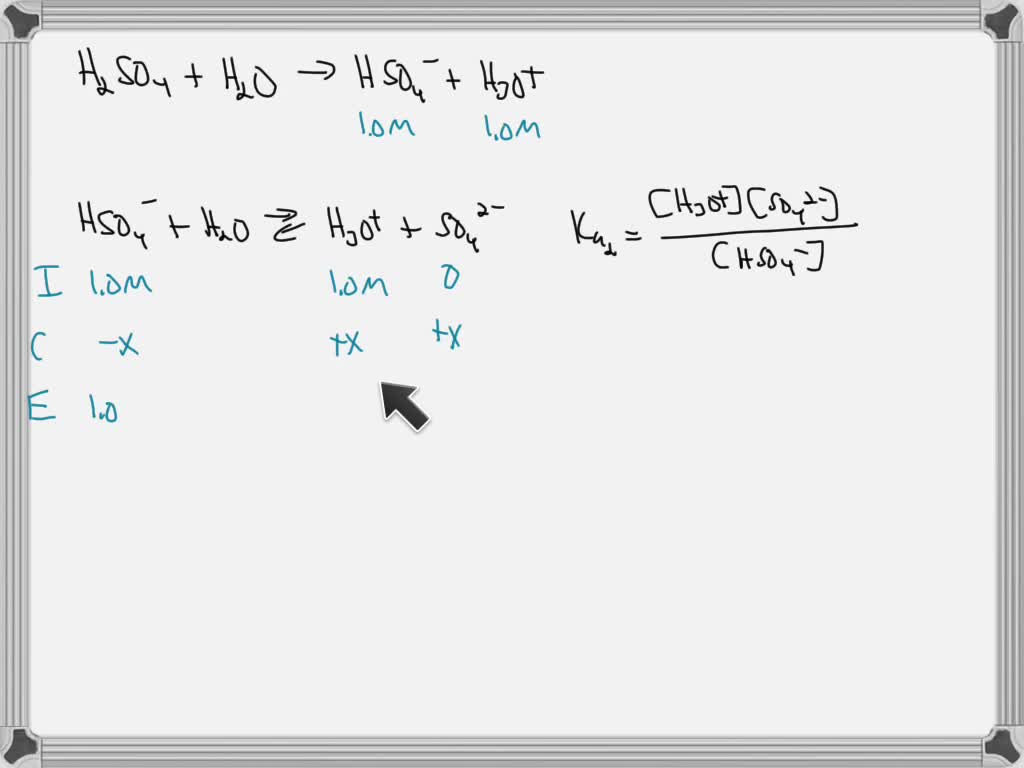
SOLVED Calculate the pH and pOH of 0.3M sulfuric acid (H2SO4) solution
Ph of a solution is the negative logarithm of concentration of $ {h^ + }\\; Sulphuric acid is reasonably treated as a strong diprotic acid that gives stoichiometric [h 3o +] and [so 42−] on dissolution. The correct ph for a 0.45m solution of h2so4 is 0.95. Thus [ h 3 o +] = 2 ×. What is the ph.
[ANSWERED] Calculate the hydronium ion concentration and the pH at the
The correct ph for a 0.45m solution of h2so4 is 0.95. What is the ph of $ 0.45m $ $ {h_2}s{o_4} $ ?. The ph of a 0.45m solution of h₂so₄ is calculated by first determining the concentration of h⁺ in the solution, then applying the ph formula. Thus [ h 3 o +] = 2 ×. Matching this value.
SOLVED a) Calculate the pH of a 2.0 M aqueous solution of H2SO4.b
Matching this value to the choices provided, the closest option is d. The correct ph for a 0.45m solution of h2so4 is 0.95. The ph calculator can determine the ph from h⁺ molar concentration, or ka, and the concentration of a solution. Sulphuric acid is reasonably treated as a strong diprotic acid that gives stoichiometric [h 3o +] and [so.
Ph Poh Map
Thus [ h 3 o +] = 2 ×. Sulphuric acid is reasonably treated as a strong diprotic acid that gives stoichiometric [ h 3 o +] and [ s o 4 2 −] on dissolution. Ph of a solution is the negative logarithm of concentration of $ {h^ + }\\; Matching this value to the choices provided, the closest.
46+ calculate the ph of a 2.0 m solution of h2so4 MorvenIyada
The correct ph for a 0.45m solution of h2so4 is 0.95. Thus [ h 3 o +] = 2 ×. The ph calculator can determine the ph from h⁺ molar concentration, or ka, and the concentration of a solution. Matching this value to the choices provided, the closest option is d. The ph of a 0.45m solution of h₂so₄ is.
What is the pH of 0.001 M H2SO4? ECHEMI
What is the ph of $ 0.45m $ $ {h_2}s{o_4} $ ?. The ph of a 0.45m solution of h₂so₄ is calculated by first determining the concentration of h⁺ in the solution, then applying the ph formula. Sulphuric acid is reasonably treated as a strong diprotic acid that gives stoichiometric [ h 3 o +] and [ s o 4.
Solved pH of Aqueous Sulfuric Acid Calculate the pH of a
The correct ph for a 0.45m solution of h2so4 is 0.95. The ph of a 0.45m solution of h₂so₄ is calculated by first determining the concentration of h⁺ in the solution, then applying the ph formula. Sulphuric acid is reasonably treated as a strong diprotic acid that gives stoichiometric [h 3o +] and [so 42−] on dissolution. Thus [ h.
Calculate the ph of 0.5 n h2so4
The ph of a 0.45m solution of h₂so₄ is calculated by first determining the concentration of h⁺ in the solution, then applying the ph formula. What is the ph of $ 0.45m $ $ {h_2}s{o_4} $ ?. Sulphuric acid is reasonably treated as a strong diprotic acid that gives stoichiometric [ h 3 o +] and [ s o 4.
SOLVED A solution contains 0.250 M HA (Ka = 1.0x106) and 0.45 M NaA
Ph of a solution is the negative logarithm of concentration of $ {h^ + }\\; The ph of a 0.45m solution of h₂so₄ is calculated by first determining the concentration of h⁺ in the solution, then applying the ph formula. Thus [ h 3 o +] = 2 ×. Sulphuric acid is reasonably treated as a strong diprotic acid that.
Matching This Value To The Choices Provided, The Closest Option Is D.
The ph calculator can determine the ph from h⁺ molar concentration, or ka, and the concentration of a solution. Sulphuric acid is reasonably treated as a strong diprotic acid that gives stoichiometric [h 3o +] and [so 42−] on dissolution. Sulphuric acid is reasonably treated as a strong diprotic acid that gives stoichiometric [ h 3 o +] and [ s o 4 2 −] on dissolution. The correct ph for a 0.45m solution of h2so4 is 0.95.
The Ph Of A 0.45M Solution Of H₂So₄ Is Calculated By First Determining The Concentration Of H⁺ In The Solution, Then Applying The Ph Formula.
Ph of a solution is the negative logarithm of concentration of $ {h^ + }\\; What is the ph of $ 0.45m $ $ {h_2}s{o_4} $ ?. Thus [ h 3 o +] = 2 ×.

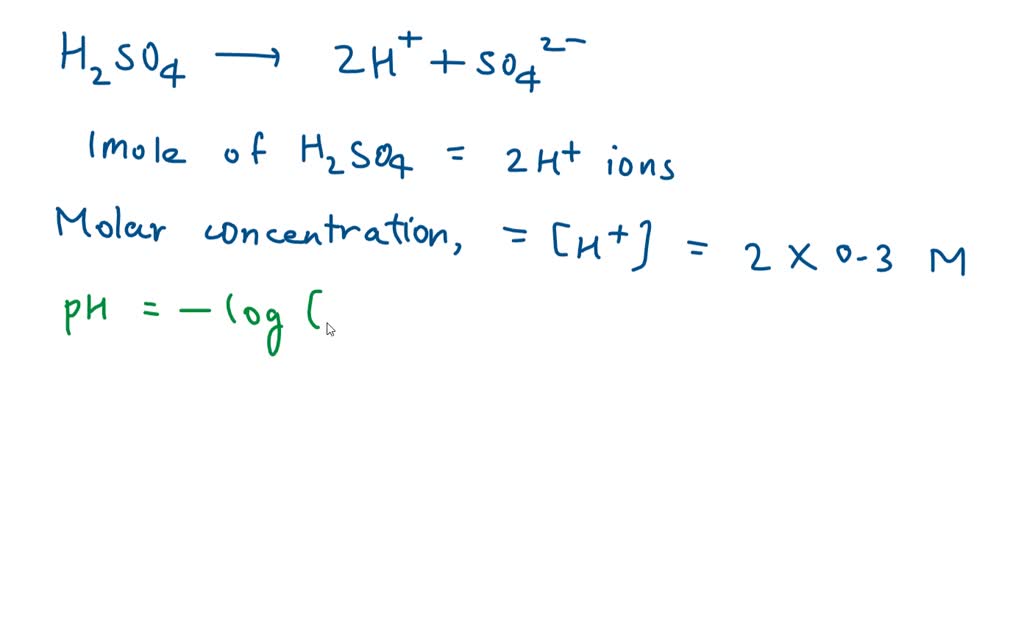
![[ANSWERED] Calculate the hydronium ion concentration and the pH at the](https://media.kunduz.com/media/sug-question-candidate/20210324165615775777-2336372.jpg?h=512)