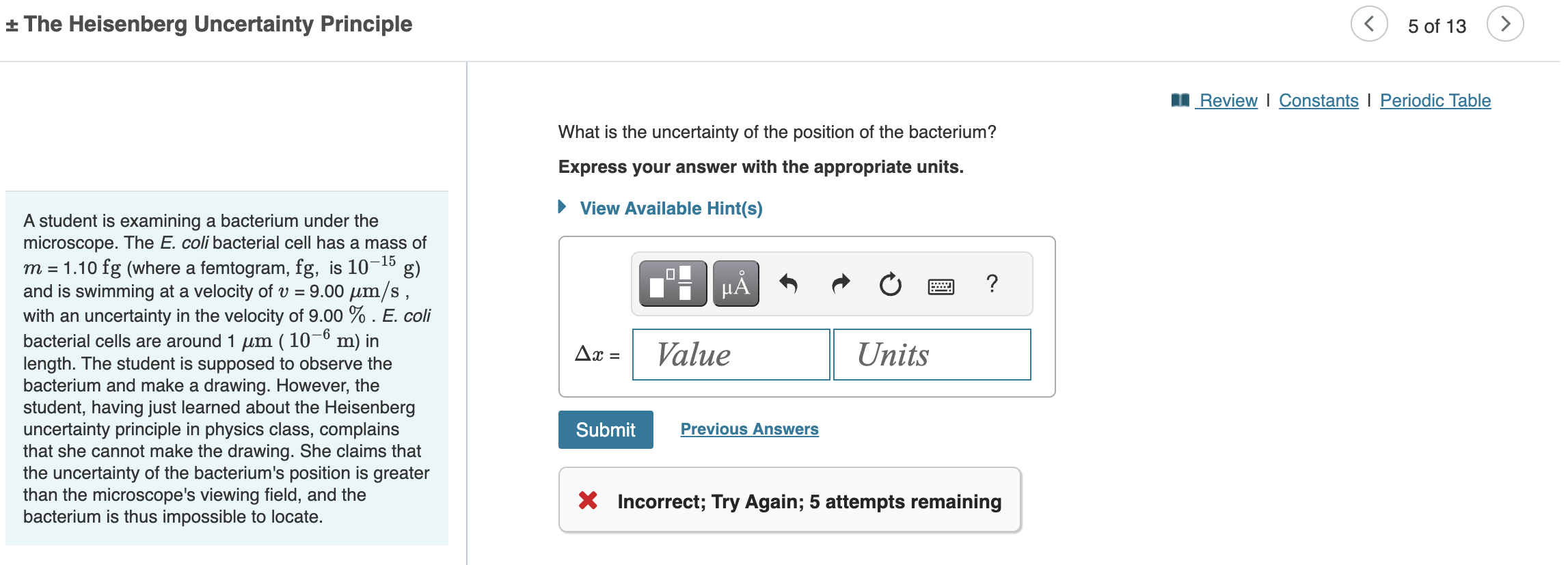
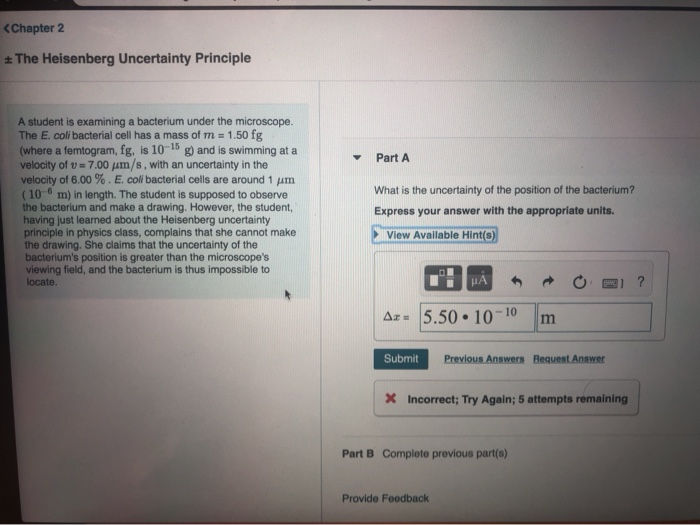
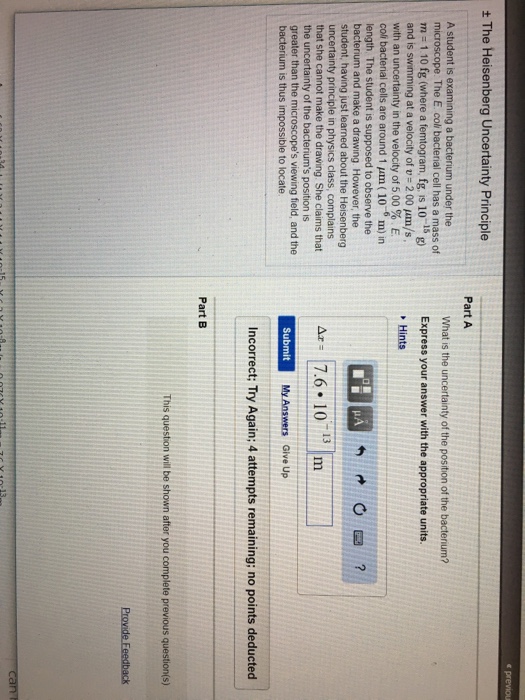
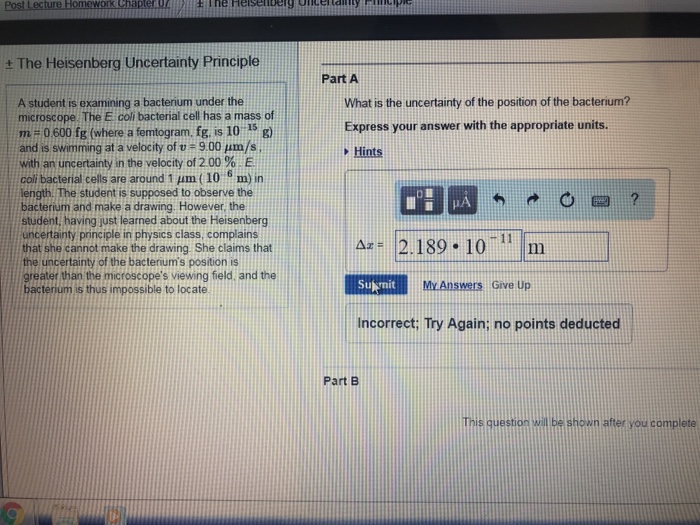
What Is The Uncertainty Of The Position Of The Bacterium
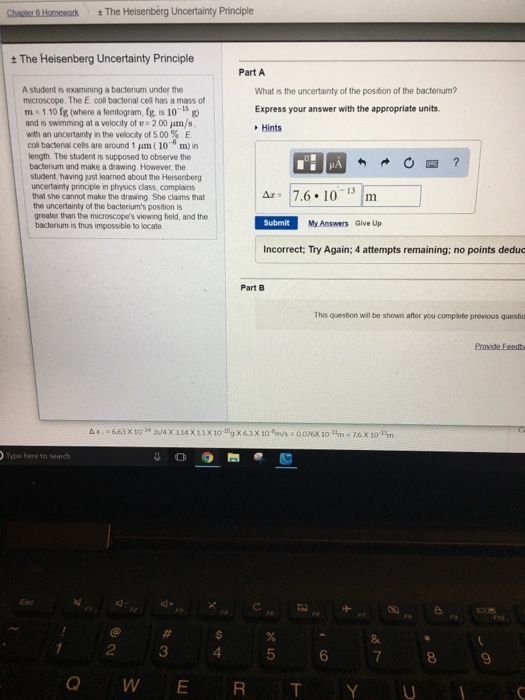
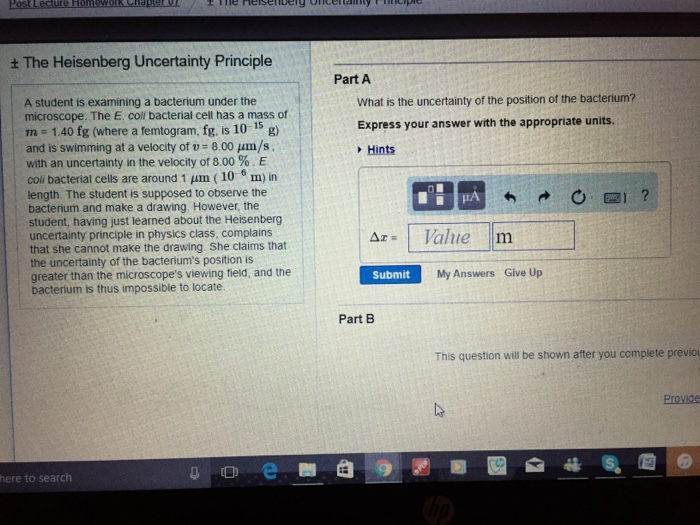
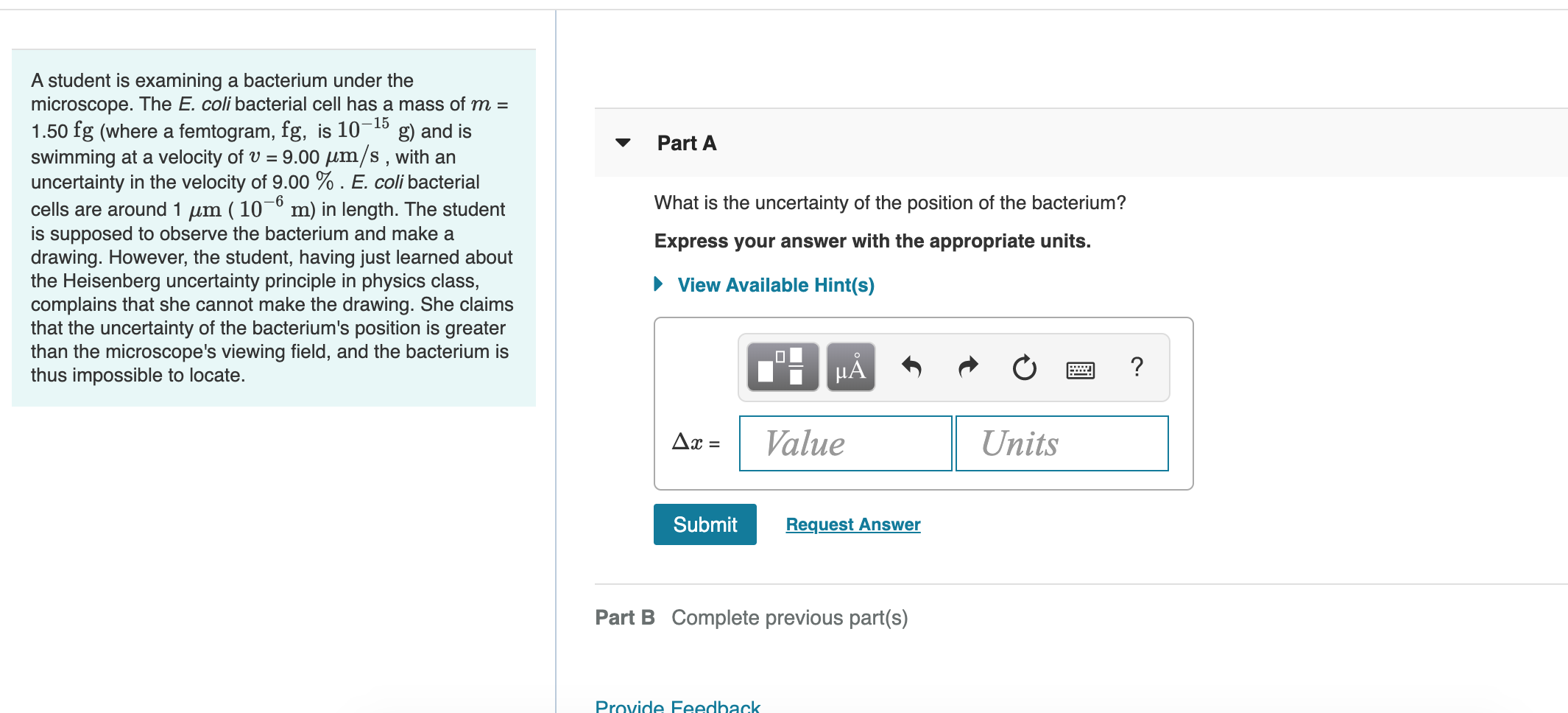
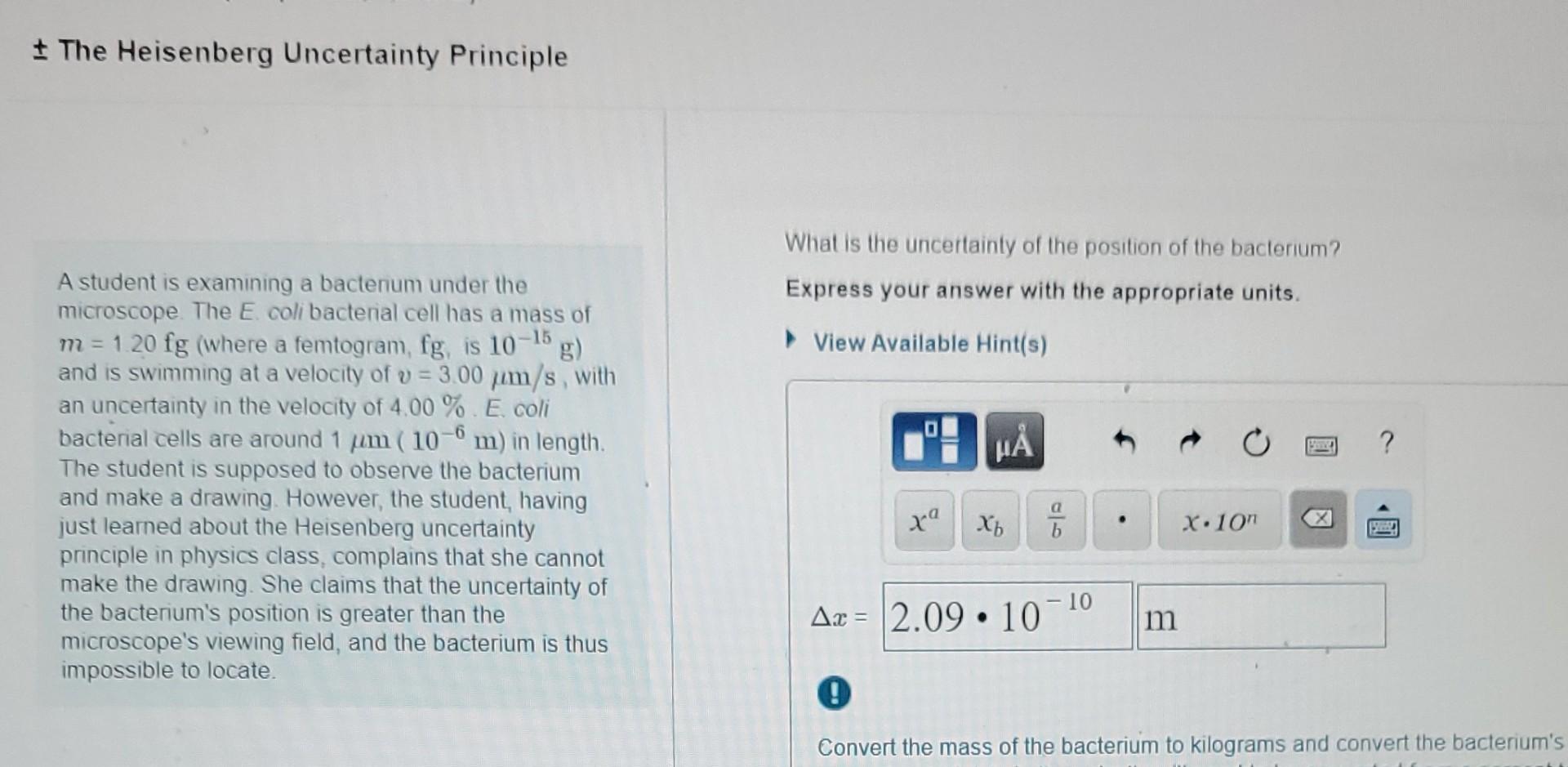
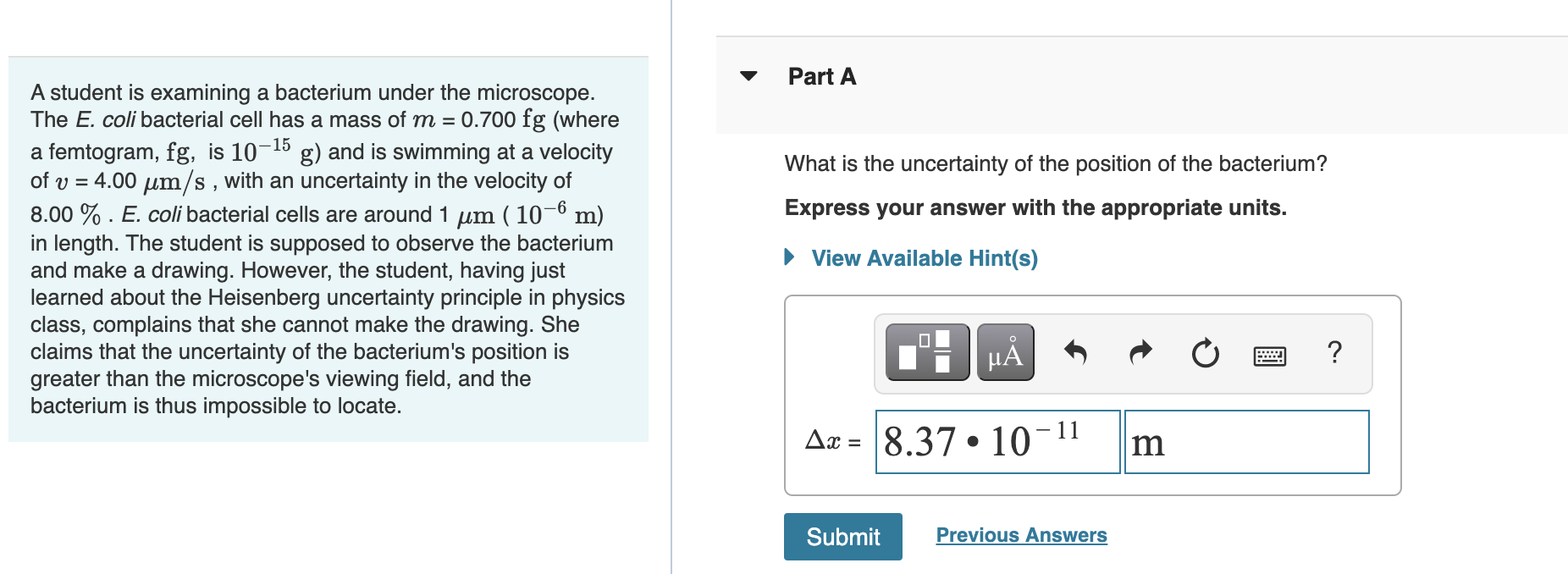
What Is The Uncertainty Of The Position Of The Bacterium - Based on what you know about the heisenberg uncertainty principle, is the student correct? Coli bacterial cell has a mass of $m = \pu{0.100 fg}$ (where a femtogram, $\pu{fg}$, is $\pu{10^{−15} g}$) and is swimming. What is the uncertainty of the position of the bacterium? Uncertainty in bacterial position refers to the inherent variability in the exact location of a bacterium within a given space. The heisenberg uncertainty principle is a fundamental principle in quantum mechanics that states that it is impossible to know the exact. Uncertainty as it relates to the heisenberg uncertainty principle in practice only. The uncertainty in the position of a bacterium is governed by the heisenberg uncertainty principle, which states that there is a. According to the heisenberg's uncertainty principle the exact position() and the exact momentum() of a particle are difficult to find with great accuracy.
According to the heisenberg's uncertainty principle the exact position() and the exact momentum() of a particle are difficult to find with great accuracy. The uncertainty in the position of a bacterium is governed by the heisenberg uncertainty principle, which states that there is a. Uncertainty in bacterial position refers to the inherent variability in the exact location of a bacterium within a given space. Coli bacterial cell has a mass of $m = \pu{0.100 fg}$ (where a femtogram, $\pu{fg}$, is $\pu{10^{−15} g}$) and is swimming. What is the uncertainty of the position of the bacterium? Based on what you know about the heisenberg uncertainty principle, is the student correct? The heisenberg uncertainty principle is a fundamental principle in quantum mechanics that states that it is impossible to know the exact. Uncertainty as it relates to the heisenberg uncertainty principle in practice only.
Coli bacterial cell has a mass of $m = \pu{0.100 fg}$ (where a femtogram, $\pu{fg}$, is $\pu{10^{−15} g}$) and is swimming. Uncertainty as it relates to the heisenberg uncertainty principle in practice only. According to the heisenberg's uncertainty principle the exact position() and the exact momentum() of a particle are difficult to find with great accuracy. What is the uncertainty of the position of the bacterium? The heisenberg uncertainty principle is a fundamental principle in quantum mechanics that states that it is impossible to know the exact. The uncertainty in the position of a bacterium is governed by the heisenberg uncertainty principle, which states that there is a. Based on what you know about the heisenberg uncertainty principle, is the student correct? Uncertainty in bacterial position refers to the inherent variability in the exact location of a bacterium within a given space.
Solved ± The Heisenberg Uncertainty Principle What is the
Uncertainty as it relates to the heisenberg uncertainty principle in practice only. The uncertainty in the position of a bacterium is governed by the heisenberg uncertainty principle, which states that there is a. Uncertainty in bacterial position refers to the inherent variability in the exact location of a bacterium within a given space. Based on what you know about the.
Solved what is the uncertainty of the position of the
Uncertainty in bacterial position refers to the inherent variability in the exact location of a bacterium within a given space. The heisenberg uncertainty principle is a fundamental principle in quantum mechanics that states that it is impossible to know the exact. Uncertainty as it relates to the heisenberg uncertainty principle in practice only. The uncertainty in the position of a.
Solved ± The Heisenberg Uncertainty Principle Part A What is
What is the uncertainty of the position of the bacterium? Coli bacterial cell has a mass of $m = \pu{0.100 fg}$ (where a femtogram, $\pu{fg}$, is $\pu{10^{−15} g}$) and is swimming. The heisenberg uncertainty principle is a fundamental principle in quantum mechanics that states that it is impossible to know the exact. According to the heisenberg's uncertainty principle the exact.
Solved ± The Heisenberg Uncertainty Principle Part A What is
The heisenberg uncertainty principle is a fundamental principle in quantum mechanics that states that it is impossible to know the exact. The uncertainty in the position of a bacterium is governed by the heisenberg uncertainty principle, which states that there is a. Coli bacterial cell has a mass of $m = \pu{0.100 fg}$ (where a femtogram, $\pu{fg}$, is $\pu{10^{−15} g}$).
Solved ± The Heisenberg Uncertainty Principle Part A What is
The heisenberg uncertainty principle is a fundamental principle in quantum mechanics that states that it is impossible to know the exact. Based on what you know about the heisenberg uncertainty principle, is the student correct? Uncertainty in bacterial position refers to the inherent variability in the exact location of a bacterium within a given space. According to the heisenberg's uncertainty.
Solved ± The Heisenberg Uncertainty Principle Part A What is
Coli bacterial cell has a mass of $m = \pu{0.100 fg}$ (where a femtogram, $\pu{fg}$, is $\pu{10^{−15} g}$) and is swimming. Based on what you know about the heisenberg uncertainty principle, is the student correct? The heisenberg uncertainty principle is a fundamental principle in quantum mechanics that states that it is impossible to know the exact. Uncertainty as it relates.
Solved Part A What is the uncertainty of the position of the
Uncertainty as it relates to the heisenberg uncertainty principle in practice only. The heisenberg uncertainty principle is a fundamental principle in quantum mechanics that states that it is impossible to know the exact. Based on what you know about the heisenberg uncertainty principle, is the student correct? The uncertainty in the position of a bacterium is governed by the heisenberg.
Solved + The Heisenberg Uncertainty Principle What is the
What is the uncertainty of the position of the bacterium? Coli bacterial cell has a mass of $m = \pu{0.100 fg}$ (where a femtogram, $\pu{fg}$, is $\pu{10^{−15} g}$) and is swimming. The heisenberg uncertainty principle is a fundamental principle in quantum mechanics that states that it is impossible to know the exact. Uncertainty in bacterial position refers to the inherent.
Solved Part A What is the uncertainty of the position of the
The heisenberg uncertainty principle is a fundamental principle in quantum mechanics that states that it is impossible to know the exact. Uncertainty as it relates to the heisenberg uncertainty principle in practice only. Uncertainty in bacterial position refers to the inherent variability in the exact location of a bacterium within a given space. Coli bacterial cell has a mass of.
Solved Part A What is the uncertainty of the position of the
Based on what you know about the heisenberg uncertainty principle, is the student correct? Coli bacterial cell has a mass of $m = \pu{0.100 fg}$ (where a femtogram, $\pu{fg}$, is $\pu{10^{−15} g}$) and is swimming. The uncertainty in the position of a bacterium is governed by the heisenberg uncertainty principle, which states that there is a. What is the uncertainty.
Based On What You Know About The Heisenberg Uncertainty Principle, Is The Student Correct?
Uncertainty as it relates to the heisenberg uncertainty principle in practice only. The uncertainty in the position of a bacterium is governed by the heisenberg uncertainty principle, which states that there is a. The heisenberg uncertainty principle is a fundamental principle in quantum mechanics that states that it is impossible to know the exact. What is the uncertainty of the position of the bacterium?
Uncertainty In Bacterial Position Refers To The Inherent Variability In The Exact Location Of A Bacterium Within A Given Space.
Coli bacterial cell has a mass of $m = \pu{0.100 fg}$ (where a femtogram, $\pu{fg}$, is $\pu{10^{−15} g}$) and is swimming. According to the heisenberg's uncertainty principle the exact position() and the exact momentum() of a particle are difficult to find with great accuracy.