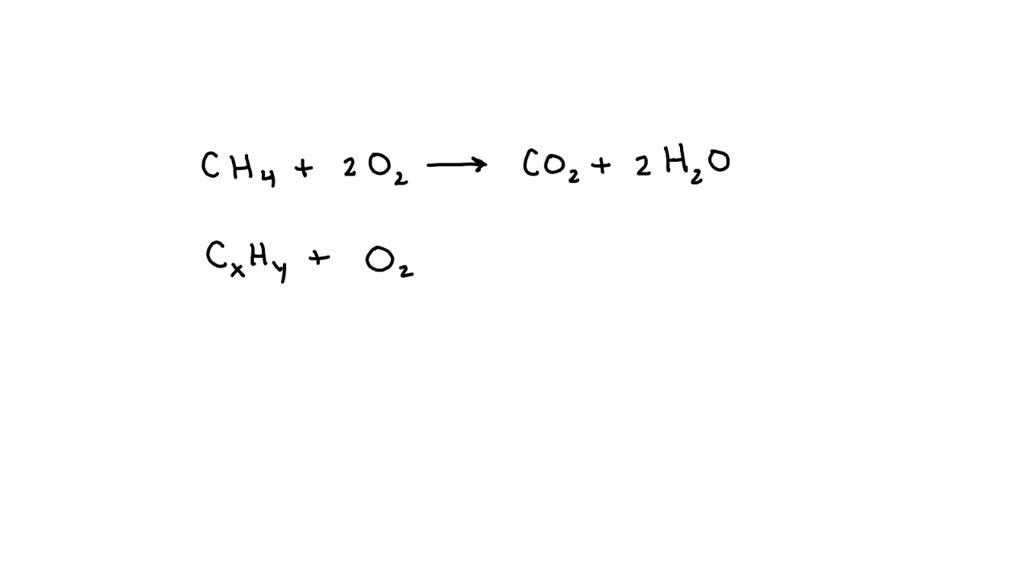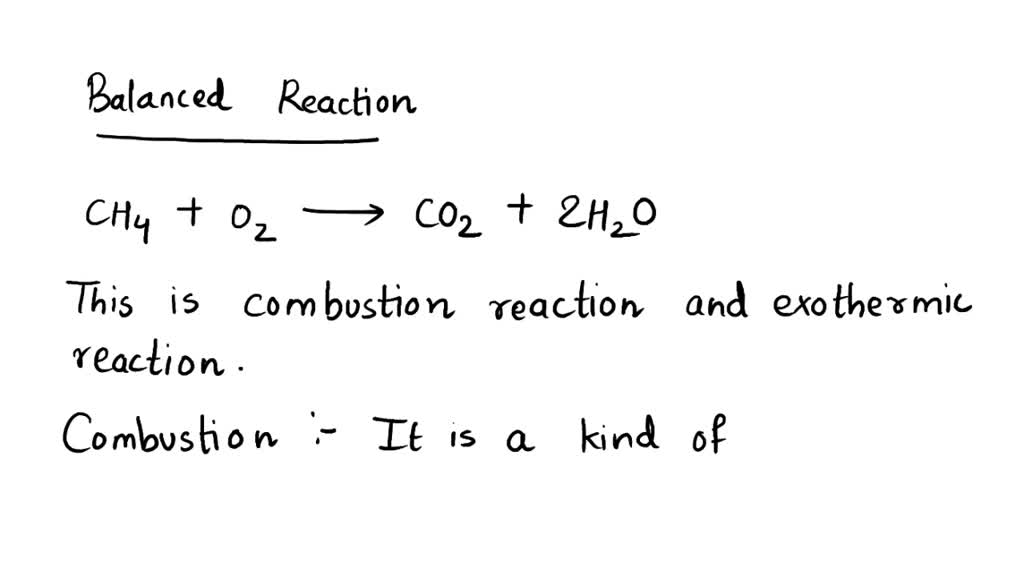What Type Of Reaction Is Ch4 2O2 Co2 2H2O 218 Kcal
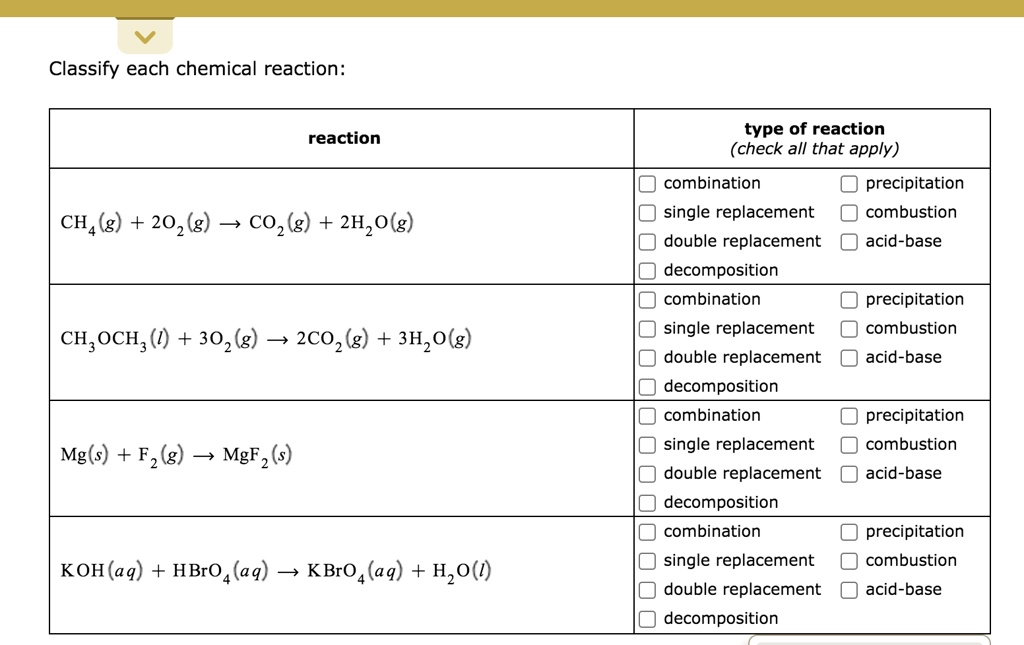
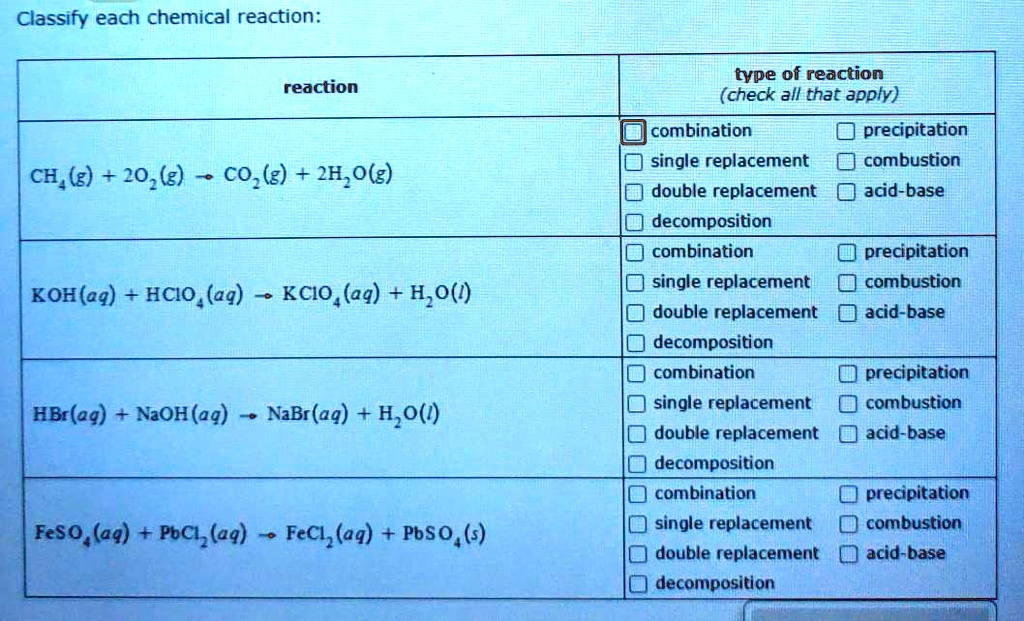
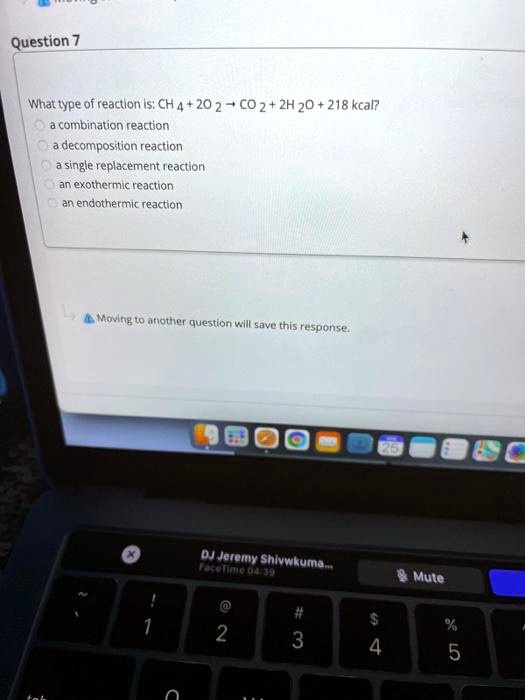
What Type Of Reaction Is Ch4 2O2 Co2 2H2O 218 Kcal - Ch4 + 2o2 → co2 + 2h2o + 218 kcal? Which reaction shows the enthalpy of formation of co2(g)? Combustion is burning in presence of. It's a combustion reaction because a hydrocarbon. What type of reaction is : It is an example of a ________ reaction. (1) ch4(g) + 2o2(g) → co2(g) + 2h2o (2) co(g) + 1/2o2(g) → co2(g) The following reaction takes place when an electric current is passed through water. This is the case with the reaction you've provided, where methane (ch4) reacts with oxygen (o2) to produce carbon dioxide (co2), water (h2o), and. What type of reaction is:
The given reaction is a combustion reaction. What type of reaction is : It is an example of a ________ reaction. Any reaction that absorbs 150 kcal of energy can be classified as ________. It's a combustion reaction because a hydrocarbon. Ch4 + 2o2 → co2 + 2h2o + 218 kcal? This is the case with the reaction you've provided, where methane (ch4) reacts with oxygen (o2) to produce carbon dioxide (co2), water (h2o), and. Ch4(g) + 2o2(g) → co2(g) + 2h2o(g) + 218 kcal is an exothermic combustion reaction. Combustion is burning in presence of. Which reaction shows the enthalpy of formation of co2(g)?
Combustion is burning in presence of. The following reaction takes place when an electric current is passed through water. Which reaction shows the enthalpy of formation of co2(g)? (1) ch4(g) + 2o2(g) → co2(g) + 2h2o (2) co(g) + 1/2o2(g) → co2(g) It's a combustion reaction because a hydrocarbon. Fe + hcl → fecl3 + h2. Ch4(g) + 2o2(g) → co2(g) + 2h2o(g) + 218 kcal is an exothermic combustion reaction. What type of reaction is: Any reaction that absorbs 150 kcal of energy can be classified as ________. It is an example of a ________ reaction.
SOLVED Classify each chemical reaction reaction type of reaction
This is the case with the reaction you've provided, where methane (ch4) reacts with oxygen (o2) to produce carbon dioxide (co2), water (h2o), and. Combustion is burning in presence of. The given reaction is a combustion reaction. It's a combustion reaction because a hydrocarbon. It is an example of a ________ reaction.
SOLVED Use the Balancing Equations Flowchart to determine the type of
(1) ch4(g) + 2o2(g) → co2(g) + 2h2o (2) co(g) + 1/2o2(g) → co2(g) Ch4(g) + 2o2(g) → co2(g) + 2h2o(g) + 218 kcal is an exothermic combustion reaction. It is an example of a ________ reaction. The given reaction is a combustion reaction. Any reaction that absorbs 150 kcal of energy can be classified as ________.
SOLVED Type of reaction (check all that apply) combination
It's a combustion reaction because a hydrocarbon. Which reaction shows the enthalpy of formation of co2(g)? Ch4 + 2o2 → co2 + 2h2o + 218 kcal? This is the case with the reaction you've provided, where methane (ch4) reacts with oxygen (o2) to produce carbon dioxide (co2), water (h2o), and. What type of reaction is :
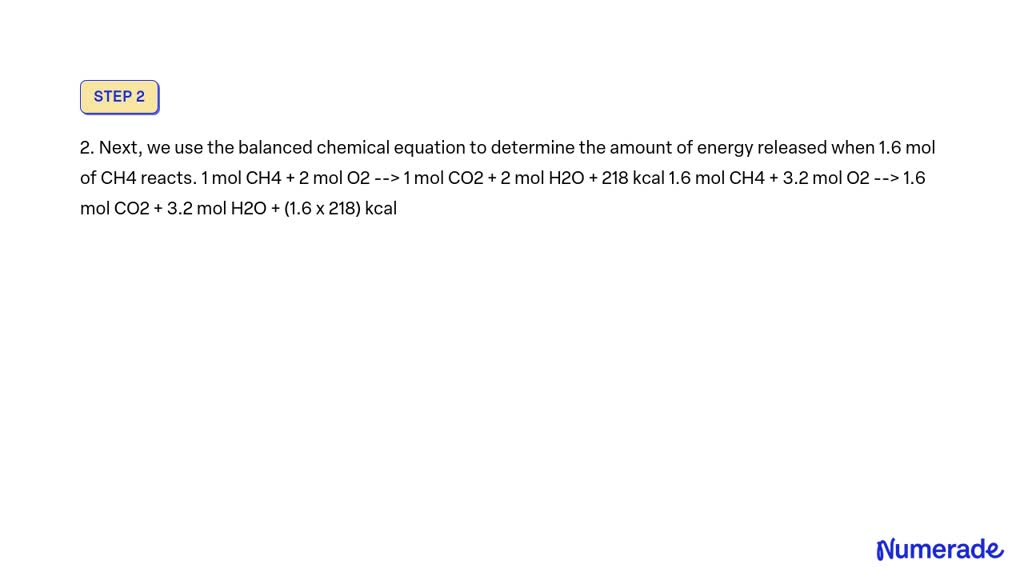
SOLVED how many kcal are produced when 32 g of CH4 react? CH4 + 2O2
What type of reaction is : The given reaction is a combustion reaction. What type of reaction is: Any reaction that absorbs 150 kcal of energy can be classified as ________. The following reaction takes place when an electric current is passed through water.
SOLVED CH4 + 2O2 > 2H2O + CO2 + 211kcal How many grams of oxygen are
Ch4(g) + 2o2(g) → co2(g) + 2h2o(g) + 218 kcal is an exothermic combustion reaction. Fe + hcl → fecl3 + h2. Any reaction that absorbs 150 kcal of energy can be classified as ________. The given reaction is a combustion reaction. Which reaction shows the enthalpy of formation of co2(g)?
SOLVED What type of reaction is CH4 + 2 O2 ® CO2 + 2 H2O + 218 kcal
Which reaction shows the enthalpy of formation of co2(g)? Ch4 + 2o2 → co2 + 2h2o + 218 kcal? It's a combustion reaction because a hydrocarbon. This is the case with the reaction you've provided, where methane (ch4) reacts with oxygen (o2) to produce carbon dioxide (co2), water (h2o), and. The following reaction takes place when an electric current is.
SOLVED What type of reaction is CH4 + O2 → CO2 + H2O + 218 kcal
What type of reaction is: Ch4(g) + 2o2(g) → co2(g) + 2h2o(g) + 218 kcal is an exothermic combustion reaction. It is an example of a ________ reaction. Any reaction that absorbs 150 kcal of energy can be classified as ________. Fe + hcl → fecl3 + h2.
SOLVED 5)What type of reaction is Ch4(g)+202(g) Co2(g)+2H2O(g)+218
(1) ch4(g) + 2o2(g) → co2(g) + 2h2o (2) co(g) + 1/2o2(g) → co2(g) It is an example of a ________ reaction. It's a combustion reaction because a hydrocarbon. What type of reaction is : What type of reaction is:
[Solved] 2. What type of reaction is CH4 2O2 → 2H2O CO2 ∆H = 218 kcal
Any reaction that absorbs 150 kcal of energy can be classified as ________. (1) ch4(g) + 2o2(g) → co2(g) + 2h2o (2) co(g) + 1/2o2(g) → co2(g) It is an example of a ________ reaction. Ch4 + 2o2 → co2 + 2h2o + 218 kcal? This is the case with the reaction you've provided, where methane (ch4) reacts with oxygen.
SOLVED Answer the following questions for the balanced reaction CO2
The given reaction is a combustion reaction. It is an example of a ________ reaction. It's a combustion reaction because a hydrocarbon. (1) ch4(g) + 2o2(g) → co2(g) + 2h2o (2) co(g) + 1/2o2(g) → co2(g) Any reaction that absorbs 150 kcal of energy can be classified as ________.
Ch4 + 2O2 → Co2 + 2H2O + 218 Kcal?
Combustion is burning in presence of. (1) ch4(g) + 2o2(g) → co2(g) + 2h2o (2) co(g) + 1/2o2(g) → co2(g) The given reaction is a combustion reaction. It's a combustion reaction because a hydrocarbon.
It Is An Example Of A ________ Reaction.
Fe + hcl → fecl3 + h2. This is the case with the reaction you've provided, where methane (ch4) reacts with oxygen (o2) to produce carbon dioxide (co2), water (h2o), and. Ch4(g) + 2o2(g) → co2(g) + 2h2o(g) + 218 kcal is an exothermic combustion reaction. Which reaction shows the enthalpy of formation of co2(g)?
What Type Of Reaction Is:
The following reaction takes place when an electric current is passed through water. What type of reaction is : Any reaction that absorbs 150 kcal of energy can be classified as ________.